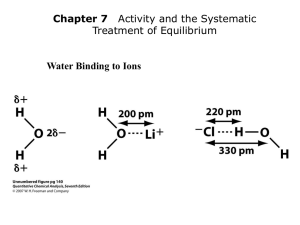
Mg 2+
advertisement

Chapter 6 Problems 6.6, 6.9, 6.15, 6.16, 6.19, 6.21, 6.24 Comments on “Lab Report & Pop Rocks” 6.6 Flip From the equations HOCl H+ + OClHOCl + OBr- HOBr + OClHOBr + OCl- HOCl + OBr- K = 3.0 x 10-8 K= 15 K’ = 1/K= 1/15 Find K for HOBr H+ + OBr— K= ? x 10-8/15 K= 3.0 K= 0.2 x 10-8 6.9 The formation of Tetrafluorethlene from its elements is highly exothermic. 2 F2 (g) + 2 C (s) F2C=CF2 (g) (a) if a mixture of F2, graphite, and C2F4 is at equilibrium in a closed container, will the reaction go to the right or to the left if F2 is added? (b) Rare bacteria … eat C2F4 and make teflon for cell walls. Will the reaction go to the right or to the left if these bacteria are added? 6.9 The formation of Tetrafluorethlene from its elements is highly exothermic. 2 F2 (g) + 2 C (s) F2C=CF2 (g) (C) will the reaction go to the right or to the left if graphite is added? (d) will reaction go left or right if container is crushed to one-eighths of original volume? (e) Does “Q” get larger or smaller if vessel is Heated? 6-15. What concentration of Fe(CN)64- is in equilibrium with 1.0 uM Ag+ and Ag4Fe(CN)6 (s). Ag4Fe(CN)6 4Ag+ + Fe(CN)64- Ksp = [Ag+]4 [Fe(CN)64-] 8.5 x 10-45 = [1.0 x 10-6]4 [Fe(CN)64-] [Fe(CN)64-] = 8.5 x 10-21 M = 8.5 zM 6-16. Cu4(OH)6(SO4) 4 Cu2+ + 6OH- + SO42I’d first set up an ICE table: I C E Cu4(OH)6(SO4) Some -x Some –x 4 Cu2+ + 4x + 4x + 6 OH 1.0 x 10-6 M Fixed at 1.0 x 10-6 M Fixed at 1.0 x 10-6 M + SO42+x +x Ksp = [Cu2+]4 [OH-]6 [SO42-] = 2.3 x 10-69 Ksp = [4x]4 [1.0 x 10-6]6 [x] = 2.3 x 10-69 X = 9.75 x 10-8 M Cu2+ = 4x = 3.90 x 10-7 M Chapter 6 Chemical Equilibrium Chemical Equilibrium Equilibrium Constant Solubility product (Ksp) Common Ion Effect Separation by precipitation Complex formation Separation by Precipitation Separation by Precipitation Complete separation can mean a lot … we should define complete. Complete means that the concentration of the less soluble material has decreased to 1 X 10-6M or lower before the more soluble material begins to precipitate Separation by Precipitation EXAMPLE: Can Fe+3 and Mg+2 be separated quantitatively as hydroxides from a solution that is 0.10 M in each cation? If the separation is possible, what range of OH- concentrations is permissible. Add OH- Mg2+ Mg2+ Fe3+ Fe3+ Fe3+ 3+ Fe 2+ Mg2+ Mg Mg2+ Mg2+ Fe3+ 3+ Fe 3+ Mg2+ 2+ Fe Mg Fe3+ Fe3+ Fe3+ Mg2+ 2+ Mg2+ Mg Fe3+ Fe3+ Mg2+ Mg2+ Mg2+ Mg2+ Mg2+ Mg2+ Mg2+ Mg2+ Mg Mg2+ Fe3+ @ equilibrium What is the [OH-] ^when this happens Mg2+ 2+ Fe(OH)3(s) EXAMPLE: Separate Iron and Magnesium? Ksp = [Fe+3][OH-]3 = 2 X 10-39 Ksp = [Mg+2][OH-]2 = 7.1 X 10-12 Assume [Fe+3]eq = 1.0 X 10-6M when “completely” precipitated. What will be the [OH-] @ equilibrium required to reduce the [Fe+3] to [Fe+3] = 1.0 X 10-6M ? Ksp = [Fe+3][OH-]3 = 2 X 10-39 EXAMPLE: Separate Iron and Magnesium? Ksp = [Fe+3][OH-]3 = 2 X 10-39 (1.0 X 10-6M)*[OH-]3 = 2 X 10-39 [ OH 3 ] 2 10 [ OH ] 1 . 3 10 33 11 Dealing with Mg2+ Find [OH-] to start precipitating Mg2+ • Conceptually – •Will assume a minimal amount of Mg2+ will precipitate and determine the respective concentration of OH- Evaluate Q • If •Q>K •Q<K •Q=K “Left” “Right” “Equilibrium” Mg2+ Mg2+ Mg2+ Mg2+ Mg2+ Mg2+ Mg2+ Mg2+ Mg 2+ Mg2+ Mg2+ Fe3+ @ equilibrium -11 [OH-]^ = 1.3 x 10 Is this [OH-] (that is in solution) great enough to start precipitating Mg2+? Fe(OH)3(s) Mg2+ Mg2+ Mg2+ Mg2+ Mg2+ Mg2+ Mg2+ Mg2+ Mg 2+ Mg2+ Mg2+ Fe3+ @ equilibrium -11 [OH-]^ = 1.3 x 10 Is this [OH-] (that is in solution) great enough to start precipitating Mg2+? Fe(OH)3(s) EXAMPLE: Separate Iron and Magnesium? What [OH-] is required to begin the precipitation of Mg(OH)2? Really, Really close to 0.1 M +2 [Mg ] = 0.10 M [Mg2+]eq = 0.09999999999999999 M Ksp = (0.10 M)[OH-]2 = 7.1 X 10-12 [OH-] = 8.4 X 10-6M EXAMPLE: Separate Iron and Magnesium? @ equilibrium [OH-] to ‘completely’ remove Fe3+ ^ -11 = 1.3 X 10 M [OH-] to start removing Mg2+ = 8.4 X 10-6M “All” of the Iron will be precipitated b/f any of the magnesium starts to precipitate!! EXAMPLE: Separate Iron and Magnesium? Q vs. K Mg(OH)2(s) Mg2+ + 2OH- Ksp = [Mg2+][OH-]2 = 7.1 X 10-12 Q = [0.10 M ][1.3 x 10-11 ]2 = 1.69 x 10-23 Q<K Reaction will proceed to “Right” Dealing with Mg2+ Find [OH-] to start precipitating Mg2+ • Conceptually – •Will assume a minimal amount of Mg2+ will precipitate and determine the respective concentration of OH- NO PPT Evaluate Q • If •Q>K •Q<K •Q=K “Left” “Right” “Equilibrium” “Real Example” Consider a 1 liter solution that contains 0.3 M Ca2+ and 0.5 M Ba2+. Can you separate the ions by adding Sodium Sodium Sodium Sodium Sodium Sodium Carbonate? Chromate ? Fluoride? Hydroxide? Iodate? Oxylate? An example Consider Lead Iodide PbI2 (s) Pb2+ + 2I- Ksp = 7.9 x 10-9 What should happen if I- is added to a solution? Should the solubility go up or down? Complex Ion Formation Complex Formation complex ions (also called coordination ions) Lewis Acids and Bases acid => electron pair acceptor (metal) base => electron pair donor (ligand) I- Pb2+ I- Pb2+ I- Pb2+ I- Pb2+ I- II- I- I- I- I- IPb2+ I- I- I- Pb2+ Pb2+ - - 2+ I- I- II- I- I- II- Pb2+ I- 2+ Pb - - I- Pb-2+ 2+ Pb I I 2+ -Pb - 2+ 2+ I- I- I- II- I- I- I- I- I- I- IPb2+ I- I- I- I- II- II- 2+ Pb - - I- Pb-2+ 2+ Pb I I 2+ -Pb - 2+ 2+ II- I- I- I- I- I- II- Pb2+ I- I- I- I- I- II- II- 2+ Pb - - I- Pb-2+ 2+ Pb I I 2+ -Pb - 2+ 2+ Effects of Complex Ion Formation on Solubility Consider the addition of I- to a solution of Pb+2 ions Pb2+ + I- <=> PbI+ K1 [ PbI [ Pb 2 ] 1 . 0 x10 ][ I ] PbI+ + I- <=> PbI2 K2 = 1.4 x 101 PbI2 + I- <=> PbI3- K3 =5.9 PbI3+ I- <=> PbI42- K4 = 3.6 2 Effects of Complex Ion Formation on Solubility Consider the addition of I- to a solution of Pb+2 ions Pb2+ + I- <=> PbI+ K1 [ PbI [ Pb 2 ] 1 . 0 x10 ][ I ] PbI+ + I- <=> PbI2 K2 = 1.4 x 101 Pb2+ + 2I- <=> PbI2 K’ =? Overall constants are designated with b This one is b2 2 Protic Acids and Bases Section 6-7 Question Can you think of a salt that when dissolved in water is not an acid nor a base? Can you think of a salt that when dissolved in water IS an acid or base? Protic Acids and Bases Salts Consider Ammonium chloride Can ‘generally be thought of as the product of an acid-base reaction. NH4+Cl- (s) NH4+ + Cl- From general chemistry – single positive and single negative charges are STRONG ELECTROLYTES – they dissolve completely into ions in dilute aqueous solution Protic Acids and Bases Conjugate Acids and Bases in the B-L concept CH3COOH + H2O CH3COO- + H3O+ acid + base <=> conjugate base + conjugate acid conjugate base => what remains after a B-L acid donates its proton conjugate acid => what is formed when a B-L base accepts a proton Question: Calculate the Concentration of H+ and OH- in Pure water at 250C. EXAMPLE: Calculate the Concentration of H+ and OH- in Pure water at 250C. Initial Change Equilibrium H2O H+ + OH- liquid - - -x +x +x +x +x Liquid-x Kw = [H+][OH-] = 1.01 X 10-14 KW=(X)(X) = 1.01 X 10-14 (X) = 1.00 X 10-7 Example Concentration of OHif [H+] is 1.0 x 10-3 M @ 25 oC? “From now on, Kw = assume the to 1 x 10-14 = [1 x 10-3][OH-] temperature be 25oC unless 1 x 10-11 = [OH-] otherwise stated.” [H+][OH-] pH ~ -3 -----> ~ +16 pH + pOH = - log Kw = pKw = 14.00 Is there such a thing as Pure Water? In most labs the answer is NO Why? CO2 + H2O HCO3- + H+ A century ago, Kohlrausch and his students found it required to 42 consecutive distillations to reduce the conductivity to a limiting value. 6-9 Strengths of Acids and Bases Strong Bronsted-Lowry Acid A strong Bronsted-Lowry Acid is one that donates all of its acidic protons to water molecules in aqueous solution. (Water is base – electron donor or the proton acceptor). HCl as example Strong Bronsted-Lowry Base Accepts protons from water molecules to form an amount of hydroxide ion, OH-, equivalent to the amount of base added. Example: NH2- (the amide ion) Weak Bronsted-Lowry acid One that DOES not donate all of its acidic protons to water molecules in aqueous solution. Example? Use of double arrows! Said to reach equilibrium. Weak Bronsted-Lowry base Does NOT accept an amount of protons equivalent to the amount of base added, so the hydroxide ion in a weak base solution is not equivalent to the concentration of base added. example: NH3 Common Classes of Weak Acids and Bases Weak Acids carboxylic acids ammonium ions Weak Bases amines carboxylate anion Weak Acids and Bases HA Ka H+ + A- Ka Ka’s ARE THE SAME [ H ][ A ] [ HA ] HA + H2O(l) H3O+ + A Ka [ H 3 O ][ A ] [ HA ] Weak Acids and Bases B + H2O Kb Kb [ BH BH+ + OH ][ OH [B] ] Relation Between Ka and Kb Relation between Ka and Kb Consider Ammonia and its conjugate base. NH3 + H2O NH4+ + H2O H2O + H2O Ka Kb NH4+ + OH- Ka [ NH Kb [ NH 3 ][ H 3 O ] 4 ][ OH ] [ NH 3 ] NH3 + H3O+ OH- + H3O+ [ NH K 4 ] [ NH 3 ][ H 3 O ] [ NH 4 ][ OH ] K [ NH 3 ] [ NH 34 ] w [ H O ] [ OH ] Example The Ka for acetic acid is 1.75 x 10-5. Find Kb for its conjugate base. Kb Kw = Ka x Kb Kb 1 . 0 10 Kw Ka 14 1 . 75 10 5 5 . 7 10 10 1st Insurance Problem Challenge on page 120