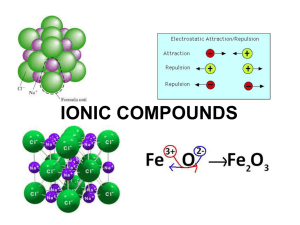
Ionic Compounds
advertisement

Ionic Compounds Chapter 8 Forming Chemical Bonds Chemical Bond: The force that holds two atoms together. Valence Electrons Opposite forces attract Octet Rule Positive Ions Lose e- becomes cation. Atoms can lose electrons to become more stable. Na+1 is more stable than Na. Negative Ions Gain e- to become anions Gain electrons to gain more stable e- config Cl-1 is more stable than Cl Formation of Ionic Bond Ionic Bond: electrostatic force that holds oppositely charged particles together in an ionic compound. Occurs between metals and nonmetals. Called ionic compounds. Formation of Ionic Bond NaCl, 1 to 1 ratio of Na and Cl Na+1 and Cl-1 combine Oxidation numbers = 0 MgCl? CaO Properties of Ionic Compounds + and – ions in regular pattern Forms crystals EX: salt Crystal lattice forms Strong bonds between them Have ↑ melting point and boiling point If aqueous solution can conduct electricity electrolyte. Properties of Ionic Compounds Formation of ionic compounds is almost always exothermic. Lattice energy: amount of energy required to separate ions of an ionic compound. Smaller ions have more negative lattice energy. EX: LiCl more negative NaCl Larger charges have larger lattice energy. EX: MgO more negative MgCl Formulas for Ionic Compounds Formula Unit: Simples ratio of ions in ionic compound Know your oxidation states for monatomic metals and nonmetals Also know your oxidation states for polyatomic metals and nonmetals Group 1 Oxidation number 1+ 2 2+ 15 3- 16 2- 17 3- Oxidation Numbers Transition metals have multiple oxidation numbers. Oxidation number: # of e- transferred from an element to form an ion. Naming Ionic Compounds 1. 2. 3. Cation always named first. Cation name does not change. (Na1+, sodium) Ex: NaCl = sodium chloride Anion named by taking root and adding –ide. (O2-, oxide) Ex: CaO = Calcium Oxide Naming Ionic Compounds 4. 5. Transition Metals with multiple oxidations must include their oxidation state in the name of the compound. [Fe2+, Iron (II)] EX: FeO = Iron (II) oxide EX: Fe2O3 = Iron (III) oxide If an atom contains a polyatomic ion simply name that ion. Know oxidation states of polyatomic ions. EX: NaOH = Sodium Hydroxide EX: AgNO3 = Silver Nitrite Naming Ionic Compounds Older method of naming transition metals Root word followed by –ous, for lower oxidation, or –ic, higher oxidation EX: Cuprous ion = Copper (I) EX: Cupric ion = Copper (II) EX: Ferrous ion = Iron (II) EX: Ferric ion = Iron (III) Naming Ionic Compounds Determine the cation and anion of the given formula Does the cation have only one oxidation number Yes Write name of cation and then write name of anion No Write the name of the cation followed by Roman numeral to represent the charge Next write the anion. Metallic Bonds and Properties of Metals Metals do not bond ironically Form electron sea Electrons are not held by 1 atom Delocalized e-, free to move Metallic bond: attraction of metallic cation for delocalized e-. Metallic Properties Very different melting points. Hg -38 C, W 3422 C. Ductile and malleable. Delocalized e- cause metals to be good conductors. Mobile e- interact with light releasing photons causing luster. Metal Alloys Alloy: mixture of elements that have metallic properties. EX: steel, Brass Substantial Alloy: atoms of original metallic solid replaced by other metal atoms of similar size Interstitial Alloy: small holes in a metallic crystal are filled with smaller atoms