Opioid Analgesics & Antagonists
advertisement

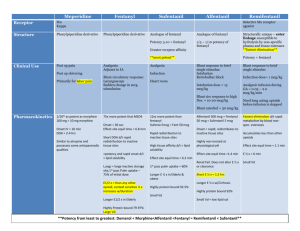
By Bohlooli S. PhD School of Medicine, Ardabil University of Medical Sciences Introduction Opium poppy is the source of crude opium Sertürner in 1803 isolated morphine Naming it after Morpheus, the Greek god of dreams Opioid analgesics is a widely used term for: Natural, semi-synthetic, synthetic Endogenous peptides Source Opium, the source of morphine, is obtained from the poppy, Papaver somniferum and P album Opium contains many alkaloids, the principle one being morphine, which is present in a concentration of about 10% Classification & Chemistry Opioid drugs include: Full agonists Morphine Partial agonists Codeine Antagonists Naloxone Chemical structure Chemistry Phenanthrenes Morphine, hydromorphone, and oxymorphone Codeine,oxycodone, dihydrocodeine, and hydrocodone Phenylheptylamines Methadone Propoxyphene Phenylpiperidines Fentanyl, sufentanil, alfentanil, and remifentanil Diphenoxylate and its metabolite, difenoxin Loperamide Morphinans Chemistry; Opioids with Mixed Receptor Actions Phenanthrenes Nalbuphine , Buprenorphine Morphinans Butorphanol Benzomorphans Pentazocine Miscellaneous Tramadol, Tapentadol Opioid Receptor Subtypes, Their Functions, and Their Endogenous Peptide Affinities Receptor Subtype Functions Endogenous Opioid Peptide Affinity (mu) Supraspinal and spinal analgesia; sedation; inhibition of respiration; slowed gastrointestinal transit; modulation of hormone and neurotransmitter release Endorphins > enkephalins > dynorphins (delta) Supraspinal and spinal analgesia; modulation of hormone and neurotransmitter release Enkephalins > endorphins and dynorphins (kappa) Supraspinal and spinal analgesia; psychotomimetic effects; slowed gastrointestinal transit Dynorphins > > endorphins and enkephalins Endogenous Opioid Peptides Endorphins Drived from: prepro-opiomelanocortin Enkephalins met-enkephalin leu-enkephalin Drived from: preproenkephalin Dynorphins Drived from: preprodynorphin Endomorphins Nociceptin / Orphanin FQ Orphanin opioid-receptor-like subtype 1 (ORL1) Pharmacokinetics Generic Name Receptor Effects1 Morphine2 Approximately Equivalent Dose (mg) Oral:Parenteral Potency Ratio Duration of Maximum Analgesia Efficacy (hours) +++ + 10 Low 4–5 High Hydromorphone +++ 1.5 Low 4–5 High Oxymorphone +++ 1.5 Low 3–4 High Methadone +++ 10 High 4–6 High Meperidine +++ 60–100 Medium 2–4 High Fentanyl +++ 0.1 Low 1–1.5 High Sufentanil +++ + + 0.02 Parenteral only 1–1.5 High Alfentanil +++ Titrated Parenteral only 0.25–0.75 High Remifentanil +++ Titrated3 Parenteral only 0.054 High Pharmacokinetics Generic Name Receptor Effects1 Approximately Oral:Parenteral Equivalent Dose Potency Ratio (mg) Duration of Maximum Analgesia Efficacy (hours) Levorphanol +++ 2–3 High 4–5 High Codeine ± 30–60 High 3–4 Low Hydrocodone5 ± 5–10 Medium 4–6 Moderate Oxycodone2,6 ± 4.57 Medium 3–4 Moderate Propoxyphene (+, very weak) 60–1207 Oral only 4–5 Very low Pentazocine ± + 30–507 Medium 3–4 Moderate Nalbuphine –– ++ 10 Parenteral only 3–6 High 0.3 Low 4–8 High Parenteral only 3–4 High Buprenorphine ± Butorphanol ± –– –– +++ 2 Pharmacokinetics Absorption Distribution Metabolism Excretion Absorption Well absorbed Variable first-pass metabolism Subcutaneous, intramuscular, and oral routes- other routes: Nasal insufflation Oral mucosa via lozenges Transdermal patches Metabolism Converted to polar metabolites Morphine morphine-3-glucuronide ::neuroexcitatory morphine-6-glucuronide ::potency four to six times Accumulation can produce unexpected results Hydromorphone like morphine H3G has CNS excitatory properties Esters (eg, heroin, remifentanil) are rapidly hydrolyzed Hepatic oxidative metabolism for phenylpiperidine opioids meperidine, fentanyl, alfentanil, sufentanil Normeperidine cause seizures in renal failure Polymorphism of CYP2D6 Codeine :: no significant analgesic effect or an exaggerated response Mechanism of Action Receptor Types Based on pharmacologic criteria 1, 2 1, 2 1, 2, 3 Genetically one subtype from each of the , and receptor families Cellular Actions Closing voltage-gated Ca2+ channels on presynaptic nerve terminals Inhibit release of Glutamate, acetylcholine, norepinephrine, serotonin, and substance P Hyperpolarizing and thus inhibiting postsynaptic neurons by opening K+ channels Relation of Physiologic Effects to Receptor Type Opioid analgesics act primarily at the -opioid receptor Analgesia, euphoria, respiratory depression, and physical dependence Butorphanol and nalbuphine Preference for opioid receptors Greater analgesia in women Receptor Distribution and Neural Mechanisms of Analgesia: Transmission Receptor Distribution and Neural Mechanisms of Analgesia: Modulation Ion Channels & Novel Analgesic Targets: chronic Pain Capsaicin receptor, TRPV1 and TRPA1 P2X : purines receptor Tetrodotoxin-resistant voltage-gated sodium channel (Nav1.8)-PN3/SNS channel Lidocaine and mexiletine Ziconotide, a blocker of voltage-gated N-type calcium channels Related to marine snail toxin -conotoxin Gabapentin/Pregabalin : analogs of GABA Ketamine: NMDA antagonists Nicotine 9-tetrahydrocannabinol Tolerance and Physical Dependence Tolerance Physical dependence Withdrawal or abstinence syndrome Mechanism receptor recycling receptor uncoupling Organ System Effects of Morphine Uterus Central Nervous System Effects Cardiovascular System Gastrointestinal Tract Biliary Tract Renal Neuroendocrine Pruritus Central Nervous System Effects Degrees of Tolerance that May Develop to Some of the Effects of the Opioids. High Moderate Minimal or None Analgesia Bradycardia Miosis Euphoria, dysphoria Constipation Mental clouding Convulsions Sedation Respiratory depression Antidiuresis Nausea and vomiting Cough suppression Central Nervous System Effects Analgesia Sensory Affective (emotional) Nonsteroidal anti-inflammatory analgesic drugs Has no effect on emotional part Euphoria Pleasant floating sensation Lessened anxiety and distress Dysphoria may occure Sedation are common effects no amnesia Sleep is in the elderly Occurs more frequently phenanthrene derivatives Central Nervous System Effects Respiratory Depression Significant respiratory depression Sepressed response to a carbon dioxide challenge Influenced significantly by the degree of sensory input Most difficult clinical challenges Cough Suppression Codeine May allow accumulation of secretions Miosis Mediated by parasympathetic pathways Truncal Rigidity Intensification of tone in the large trunk muscles Nausea and Vomiting Activate the brainstem chemoreceptor trigger zone Temperature -opioid receptor agonists hyperthermia -opioid receptor agonists hypothermia Cardiovascular System Bradycardia Meperidine antimuscarinic action tachycardia Hypotension may occur Peripheral arterial and venous dilation Release of histamine Central depression of vasomotor-stabilizing mechanisms Caution in patients with decreased blood volume Gastrointestinal Tract Constipation the stomach Motility decrease Tone increase Gastric secretion of hydrochloric acid is decreased Biliary Tract Contract biliary smooth muscle biliary colic Sphincter of Oddi may constrict Other Peripheral Effects Renal Antidiuretic effect Enhanced renal tubular sodium reabsorption Increased ureteral and bladder tone Uterus May prolong labor Neuroendocrine stimulate the release of ADH, prolactin, and somatotropin inhibit the release of luteinizing hormone • Clinical Use of Opioid Analgesics • Toxicity & Undesired Effects Alternative Routes of Administration Rectal suppositories morphine and hydromorphone Transdermal patch Fentanyl Intranasal Butorphanol Buccal transmucosal Fentanyl citrate lozenge Patient-controlled analgesia (PCA) infusion device Toxicity & Undesired Effects Behavioral restlessness, tremulousness, hyperactivity (in dysphoric reactions) Respiratory depression Nausea and vomiting Increased intracranial pressure Postural hypotension accentuated by hypovolemia Constipation Urinary retention Itching around nose, urticaria (more frequent with parenteral and spinal administration) Tolerance and Dependence Does not become clinically manifest until after 2–3 weeks Tolerance to methadone develops more slowly Cross-tolerance is an extremely important But often be partial or incomplete Opioid rotation Recoupling opioid receptor ketamine Physical Dependence Signs and symptoms Rhinorrhea Lacrimation Yawning Chills Gooseflesh (piloerection) Hyperventilation Hyperthermia Mydriasis Muscular aches Vomiting Diarrhea Anxiety, and hostility Physical Dependence time of onset, intensity, and duration of abstinence syndrome depend on biologic half-life morphine or heroin, usually start within 6–10 hours methadone required several days Psychologic Dependence Euphoria, indifference to stimuli, and sedation Abdominal effects that have been likened to an intense sexual orgasm Reinforced by the development of physical dependence The Opioid Antagonists Naloxone,naltrexone, and nalmefene Methylnaltrexone bromide Alvimopan

![Shark Electrosense: physiology and circuit model []](http://s2.studylib.net/store/data/005306781_1-34d5e86294a52e9275a69716495e2e51-300x300.png)








