Brief Silicone Chemistry Review & Silicones for the Skin Care Industry
advertisement

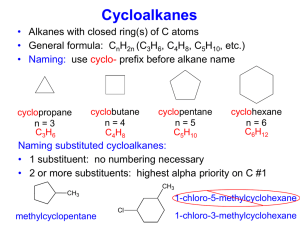
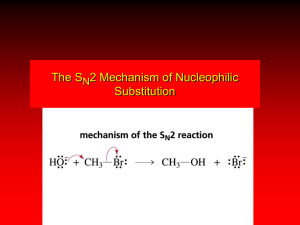
Basic Silicone Chemistry (I) Silicone Family Tree Elastomers Fluids & Emulsions Silanes Silicone Resins Dimethyl Compounds Silicone Polyethers Organo-Silicones Volatile Methyl Siloxanes Amino Silicones Si Flexibility of Siloxane Chemistry • • • • • • • Non-volatile Antifoam Slippery Water Insoluble Excellent Depth of Gloss Incompatible in Organics Durable • • • • • • • Volatile Profoam Sticky Water Soluble Shiny Compatible Transient SILICONES APPLICATIONS Dow Corning’s products and specialty materials are used by customers in virtually every major industry. Aerospace Automotive Chemicals/ Petrochemicals Construction Consumer Products Electrical/Electronics Food Processing Industrial Maintenance Production Medical Products Paints & Coatings Personal, Household & Automotive Care Pharmaceuticals Plastics Pressure-Sensitive Adhesives Textiles & Leather Silicone Nomenclature Si SILICON O SILICA O Si O O SILANES X X Si X X R SILOXANES O Si R O Silicone Nomenclature Shorthand Precursor Silanol Siloxane Structure Short hand Me Cl-Si-Cl Me Me HO-Si-OH Me Linear Structures D unit End-cap group M unit Me Me-Si-Cl Me Me Me-Si-OH Me Me Cl-Si-Cl Cl Me HO-Si-OH OH Branched Structures T unit Cl Cl-Si-Cl Cl OH HO-Si-OH OH Silica Core Q unit Me Me Me Me Me Me-Si-O-Si-O-Si-O-Si-O-Si-Me Me Me Me Me Me = Me Me Me Me-Si-(O-Si)3-O-Si-Me Me Me Me = MD3M Silicone Classifications by Physical Form (1) Fluids (hydraulic, release agents, cosmetics, heat transfer media, polishes, lubricants, damping, dry cleaning) Polymer chains of difunctional units (D) terminated with monofunctional (M) units OR cyclics (Dx) (2) Gums (high temperature heat transfer fluids, lubricants, greases, cosmetic and health care additives) Same structure as PDMS fluids, but much higher molecular weight (viscosities >1,000,000 cSt). (3) Resins (varnishes, protective coatings, release coatings, molding compounds, electronic insulation) Rigid solids based on trifunctional (T) and tetrafunctional (Q) units. Surface modification with (M) units (4) Elastomers (Heat cured and RTVs: tubing and hoses, medical implants, sealants, adhesives, surgical aids, electrical insulation, fuel resistant rubber parts, rollers, etc) Soft solids based on crosslinked SiH Fluids Raw Materials • Initial material is quartz – SiO4/2 – 26% of the Earth’s crust • Reduce to Si metal with carbon at 2500F • Dow Corning purchases silicon metal & methanol • Methanol is converted to MeCl with recycled HCl Process Chemistry of Methyl Train Me2SiCl2 MeHSiCl2 Me3SiCl Chlorosilane Mix Si MeCl Copper Catalysts H2O Me2 Hydro SiH fluid EBB Waste & Recovery Volatile Polydimethylsiloxane Fluids INCI NAME: Cyclomethicone CH3 Si - O CH3 CH3 CH3 Si n CH3 CH3 n = 3 Trimer n = 4 Tetramer n = 5 Pentamer n = 6 Hexamer CH3 O O CH3 Si Si O O Si CH3 O Si CH3 CH3 CH3 PENTAMER (D5) Volatile Polydimethylsiloxane (PDMS) Fluids R = CH3 INCI: Dimethicone R = OH INCI: Dimethiconol CH3 CH3 CH3 R - Si - O - Si - O - Si - R CH3 CH3 m CH3 When m = 0, R= CH3 called Hexamethyldisiloxane or 200 Fluid,0.65 cS (.65,1, 1.5 and 2.0 cS are volatile) Properties of Siloxanes • Despite the Fact that Silicon and Carbon are both Group IV elements their chemistry is very different • Unique flexibility of Si-O bond • bond length bond angle bond energy bond barrier Si-O-Si 1.63 130 106 0.2 C-C-C 1.54 112 83 3.6 C-O-C 1.42 111 86 2.7 units angstroms degree Kcal/mol Kcal/mol Siloxane Polymers vs Carbon Polymers •Barrier to Rotation ( kcal/mole ) –Polyethylene 3.3 –Polytetrafluoroethylene 4.7 –Polydimethylsiloxane < 0.2 Key Point: Siloxane (Si-O-Si) polymers are stronger than carbon polymers, yet the polymer chains are more open and flexible Siloxane Physical Properties • Very low glass transition temperature (Tg = -120 °C) – high molecular weights but not a solid • Ability to spread out on a wide variety of substrates – silky, smooth, non-tacky, aesthetic enhancing – flowability and film forming • Lowest surface shear viscosity and low surface tension – lubricating, antifoaming, waterproofing, release properties • High gas permeability • Excellent dielectric properties • Very good thermo-oxidative stability – good chemical inertness and temperature resistance Anionic Ring Opening Equilibrations Me Me D4 Si O Me Me O KOH Si HO Me Me Si Si O Si Me Me Si K O 3 O O Me Me Me Me Me Me Me K O Si O Si O K D4 K n O Si Me O Me Me R R R Si O R Ring 10-15% : : Chain Equilibrium 85 – 90% Si O Si Me R R O Si n R Si R Me R R PDI = 2.0 K O 7 Me Me Si R R Anionic Ring Opening Equilibrations R End Blockers R Si R CH3 H3C Si R O Si R R CH3 O Si CH3 CH3 CH3 H2NCH2CH2CH2 CH3 CH3 Si O Si CH3 CH2CH2CH2NH2 CH3 O CH3 O H2N Si O CH3 O Si CH3 CH3 O NH2 Si CH3 CH3 O O Si O CH3 CH3 Anionic Ring Opening Equilibrations CH3 End Blockers H3C Si CH3 Viscosity CH3 O Si CH3 CH3 Maximum in viscosity involves incorporation of end blocker (which is less reactive than cyclic) time Living Anionic Ring Opening Polymerization H3C CH3 Si O O cyclohexane sec-Butyl – Li + H3C Si Si O H3C CH3 H3C CH3 CH2 CH Me CH3 Si O 2 CH3 CH3 Si O Li Me D3 10% THF D3 R CH3 H3C CH2 CH Me CH3 Si CH3 O Si n Cl R O Si R Si R CH3 Me CH3 R H3C CH2 CH Si O Si R Me CH3 LiCl Living anionic polymerization n Me O Li