Organic Reactions - Rosebank Progress College
advertisement

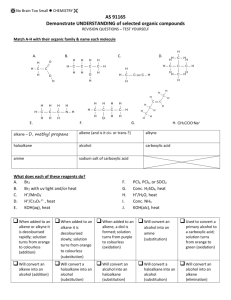
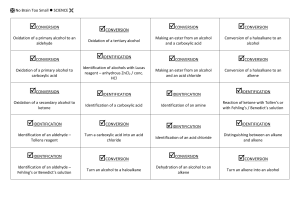
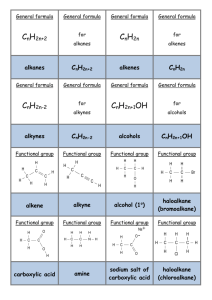
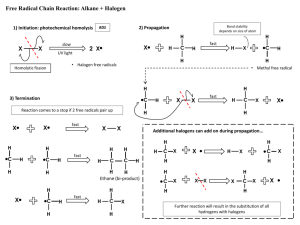
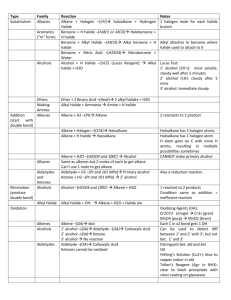
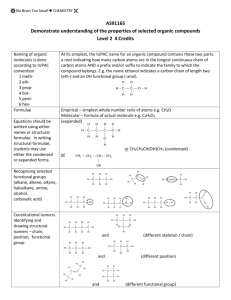
IMPORTANT ORGANIC REACTIONS Presentation created by S. Schlosz Information by N. Solomons, K. Dilraj & S. Schlosz What must you be able to do? • identify the types of reactions that hydrocarbons undergo. • explain what happens during each type of reaction. • compare the reactivity of different hydrocarbons. Reactions of Alkanes: H H H C H + Br Br H HALOGEN (as found in the B r Normally table) diatomic B r periodic + H C H H Conditions: Heat OR sunlight; Reactants: alkane + X2 (Br, Cl, I, F) Process = halogenation; Products = haloalkane + hydrogenFhalide (F ) Fluorine NOTE: F- (Fluoride) 2 This is a hydrogen halide [acid]. Cl- (Chloride) Cl (Cl2) Chlorine 2C6H14 + 19O2 → 12CO2 + 14H2O (Br2) Bromine Alkane + oxygen → carbon dioxide + water +Br energy H C H H C H H C H H C H H C H H H H H H C C C H H H H H + H C H Conditions: Heat and high pressure OR heat and catalyst Process = cracking Products = alkene(s) + alkane(s) REACTIONS OF OXIDATION (COMBUSTION) ALKANES Br- (Bromide) I- (Iodide) I (I2) Iodine H HALIDE (the state of the Halogen after it has received an electron via SUBSTITUTION bonding) Alkane becomes haloalkane ELIMINATION C H Alkane becomes alkene(s) and alkane(s) with shorter chain REACTIONS OF ALKENES NOTE: Only minor product shown. This is the CIS structure. The major product will have the TRANS structure [Cl on opposite sides of the different Carbons] H H H C + C H H Cl Cl H C H C Cl H H H H C C H H H C + C Cl HBr H H H H H C C C C H Br H H H M a jo r p ro d u ct H Process = halogenation Product = haloalkane H +/ / H H H H H C C C C H H H Br H No water present; Process = hydrohalogenation Product = haloalkane Major product: H atom attaches to the C atom already having the greater number of H atoms NOTE: This is Markovnikov’s Rule for ADDITION Reactions. ADDITION Alkene becomes alkane, alcohol or haloalkane H H H H H H C C H H NOTE: The acid must be dilute H2SO4 or H3PO4. Cannot use HNO3 & HCl as they produce gases. H C + H C + C Pt H H H C C H H H C H H H 2O H H H H H C C C C H H OH H M a jo r p ro d u ct H + / H H H H H C C C C H H H OH 150C H H Pt, Pd or Ni as catalyst Process = hydrogenation; product = alkane In presence of excess H2O and acid as catalyst Process = hydration; product = alcohol Major product: H atom attaches to the C atom already having the greater number of H atoms H Haloalkanes are important compounds that are used as anesthetics (trichloromethane), solvents and dry cleaning agents. Tetrachloroethane Trichloromethane (chloroform) Tetrachloromethane (Carbon tetrachloride) H Halo-ethane H H H H H C C C C H Br H + Na OH heat H H H H H C H H C C H H H H C C H m a jo r p ro d u ct H C Br H C H + C H H C C H H H H H + Na OH H + N aB r + H 2O HaloAlkanes undergo: H C H C H H heat H C C H H H H H C C H H H C C C H H H H H /+ C H H + N aB r + H 2O ELIMINATION Haloalkane becomes alkene m a jo r p ro d u ct Conditions: concentrated strong base (NaOH, KOH, LiOH), heat Process = dehydrohalogenation; Products = alkene + NaBr + H2O Major product: The one where the H atom is removed from the C atom with the least number of H atoms (most substituted double bond forms i.e. double bond with most alkyl groups forms) NOTE: This is VASILY SAYTZEFF’s Rule for ELIMINATION Reactions. REACTIONS OF HALOALKANES H H H H H C C C C H H Br H H + Na OH H H H H H C C C C H H OH H H + Na Br Conditions: Dilute strong base (NaOH, KOH, LiOH), mild heat Substitution - hydrolysis; Products = alcohol + NaBr (KBr or LiBr) SUBSTITUTION Haloalkane becomes alcohol H H H H H C C C C H H Br H H + H 2O H H H H H C C C C H H OH H Conditions: Add water, mild heat Substitution – hydrolysis; Products = alcohol + HBr H + HBr H H C H Primary REACTIONS OF ALCOHOLS Secondary Alcohols Tertiary / Alcohols The O-H group Alcohols + is attached to The O-H group The O-H group is attached to a Carbon is attached to a Carbon which is a Carbon is + attached to 1 which which is other Carbon attached to 2 attached to 3 SUBSTITUTION ELIMINATION other other H H Carbons REACTIONS OF ALCOHOLS Carbons H H H H C C C OH H H H2SO4 H H H H C H C C C H H C C C H H H H H + C H H H H m a jo r p ro d u c t H + H 2O H Elimination of H2O – dehydration; Products = alkene + H2O Major product: The one where the H atom is removed from the C atom with the least number of H atoms (most substituted double bond forms i.e. double bond H H C C H OH H To produce gaseous alkenes: pass alcohol over heated AlCl3 H H C C H H H H H 2O C H H Alcohol becomes haloalkane Alcohol becomes alkene H– C–C–O–H H H O H ESTERIFICATION H Acid catalysed condensation Alcohol + carboxylic acid → ester + water H2SO4 H H H – C– C–C–H O H H – C – CH– C – H H H H H C C C C O H H H H H H O + H C C H Br Reactants needed: Primary & secondary alcohols: NaBr + H2SO4 Tertiary alcohols: HBr (or HCl) H C O H H Substitution with hydrogen halide Products = haloalkane + H2O with most alkyl groups) H HBr H H H H H C O C H H H H H C C C C H H H H H C C HH HO - CH - H + H 2O O H H H H + H 2O