chemical equation
advertisement

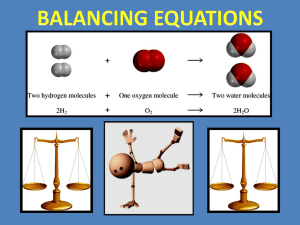
Writing Chemical Equations Parts of a Reaction ► Reactants The starting materials in a chemical reaction ► Products The substances that are formed in a chemical reaction Parts of a Reaction ►A chemical equation shows how atoms are rearranged during a reaction Reactants appear on the LEFT Products appear on the RIGHT An arrow separates the reactants from the products () Reactants Products Special Notations ► Descriptions of the compounds appear as subscripts after the compound. ► Gas: (g) ► Liquid: ► Solid: (l) (s) ► Aqueous: Dissolved in water (aq) Special Notations ► Special conditions appear with the arrow. ► Heat: Heat is added to make the reaction happen D ► Catalyst: A catalyst is added in order for the reaction to happen – The compound is listed Pt Writing a Chemical Equation Potassium oxide (K2O) and water react to form potassium hydroxide (KOH). Reactants: K2O, H2O Products: KOH K2O + H2O KOH Writing a Chemical Equation Aluminum and iron oxide (FeO) react to form aluminum oxide (Al2O3) and iron. Reactants: Al, FeO Products: Al2O3, Fe Al + FeO Al2O3 + Fe Writing a Chemical Equation Silicon dioxide (SiO2) and hydrogen fluoride (HF) react to form silicon tetrafluoride (SiF4) and water. Reactants: SiO2, HF Products: SiF4, H2O SiO2 + HF SiF4 + H2O Writing a Chemical Equation Solid sodium carbonate (Na2CO3) and aqueous hydrochloric acid (HCl) react to form carbon dioxide gas (CO2), liquid water, and aqueous sodium chloride (NaCl) Reactants: Na2CO3(s), HCl(aq) Products: CO2(g), H2O(l), NaCl(aq) Na2CO3(s) + HCl(aq) CO2(g) + H2O(l) + NaCl(aq) Writing a Chemical Equation Heating solid sodium nitrate (NaNO3) results in solid sodium nitrite (NaNO2) and gaseous oxygen (O2) Reactants: NaNO3(s) Products: NaNO2(s), O2(g) Special Conditions: Heat D NaNO3(s) NaNO2(s) + O2(g) Writing a Chemical Equation Aqueous hydrogen peroxide (H2O2) reacts to form liquid water (H2O) and oxygen gas (O2) in the presence of a platinum catalyst (Pt). Reactants: H2O2(aq) Products: H2O (l), O2(g) Special Conditions: Platinum Catalyst Pt H2O2(aq) H2O (l)+ O2(g) Balancing an Equation ► In a chemical reaction, atoms are rearranged, but they cannot be destroyed or changed. ► If an atom appears on the left side of the reaction, it must appear on the right side. Balancing an Equation K2O + H2O KOH ► In the reaction above, there are unequal numbers of atoms on either side Potassium (K): 2 atoms on the left, 1 atom on the right Oxygen (O): 2 total on the left, 1 atom on the right Hydrogen (H): 2 atoms on the left, 1 atom on the right. Balancing an Equation K2O + H2O KOH ► The reaction must be modified in order to make the atoms equal ► We CANNOT add subscripts to KOH, because that would change the compound. ► Instead, we add coefficients in front of compounds. Balancing an Equation Determine how many of each atom are present. ___ K2O + ___ H2O ___ KOH K O K K: 2 H: 2 O: 2 H O H K O H K: 1 H: 1 O: 1 Balancing an Equation There are different numbers of atoms on both sides. In order to match them up, we can add more compounds, but not atoms alone. ___ K2O + ___ H2O ___ KOH K O K K: 2 H: 2 O: 2 H O H K O H K O H K: 1 H: 1 O: 1 Balancing an Equation The number of each atom in the compound changes. ___ K2O + ___ H2O ___ KOH K O K K: 2 H: 2 O: 2 H O H K O K O K: 1 H: 1 O: 1 H H 2 2 2 Balancing an Equation Now that all the atoms are balanced, we can add up all the compounds. This is called a coefficient 1 K2O + 1 H2O 2 KOH K O K K: 2 H: 2 O: 2 H O H K O K O K: 1 H: 1 O: 1 H H 2 2 2 Balancing an Equation Determine how many of each atom are present. ___ H2 + ___ O2 ___ H2O H H H: 2 O: 2 O O H O H H: 2 O: 1 Balancing an Equation The hydrogen is okay, but the oxygen is not. Add another H2O in order to balance the oxygen. ___ H2 + ___ O2 ___ H2O H H H: 2 O: 2 O O H O H H O H H: 2 O: 1 Balancing an Equation The hydrogen is okay, but the oxygen is not. ___ H2 + ___ O2 ___ H2O H H K: 2 H: 2 O: 2 O O H O K O K: 1 H: 1 O: 1 H H 2 2 2 Balancing an Equation ___ Al + ___ FeO ___ Al2O3 + ___ Fe Al: 1 O: 1 Fe: 1 Al: 2 O: 3 Fe: 1 Balancing an Equation Begin balancing the aluminum by adding a 2 _2_ Al + ___ FeO ___ Al2O3 + ___ Fe Al: 1 O: 1 Fe: 1 Al: 2 O: 3 Fe: 1 Balancing an Equation Recount the aluminum atoms _2_ Al + ___ FeO ___ Al2O3 + ___ Fe Al: 2 O: 1 Fe: 1 Al: 2 O: 3 Fe: 1 Balancing an Equation Move on to oxygen – Balance it by placing a 3 in front of FeO, and then recount the atoms _2_ Al + _3_ FeO ___ Al2O3 + ___ Fe Al: 2 O: 3 Fe: 3 Al: 2 O: 3 Fe: 1 Balancing an Equation By adding the 3, we also changed the iron – this is the only way to balance, so we now fix the iron by adding another coefficient _2_ Al + _3_ FeO ___ Al2O3 + _3_ Fe Al: 2 O: 3 Fe: 3 Al: 2 O: 3 Fe: 3 Balancing an Equation Now everything is balanced _2_ Al + _3_ FeO ___ Al2O3 + _3_ Fe Al: 2 O: 3 Fe: 3 Al: 2 O: 3 Fe: 3 Balancing an Equation Identify the number of each atom present – Start with atoms in the fewest compounds. We tend to keep H and O last. ___ SiO2 + ___ HF ___ SiF4 + ___ H2O Si: 1 F: 1 H: 1 O: 2 Si: 1 F: 4 H: 2 O: 1 Balancing an Equation Silicon is done, so move on to fluorine. ___ SiO2 + _4_ HF ___ SiF4 + ___ H2O Si: 1 F: 4 H: 4 O: 2 Si: 1 F: 4 H: 2 O: 1 Balancing an Equation Now move on to hydrogen. Since the compound has 2 hydrogen atoms in it, we multiply to get the correct number. ___ SiO2 + _4_ HF ___ SiF4 + _2_ H2O Si: 1 F: 4 H: 4 O: 2 Si: 1 F: 4 H: 4 O: 2 Balancing an Equation Oxygen is already done. Everything is balanced. ___ SiO2 + _4_ HF ___ SiF4 + _2_ H2O Si: 1 F: 4 H: 4 O: 2 Si: 1 F: 4 H: 4 O: 2