Carbohydrate chemistry
advertisement

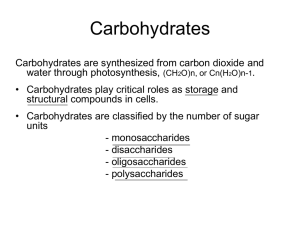
Carbohydrate chemistry Definition Nomenclature Cyclic form of sugar Optical isomerism Glycosidic link Polysaccharides Carbohydrate derivatives Definition Carbohydrate are polyhydroxy aldehydes,polyhydroxyketones or compounds that can be hydrolysed to them Monosaccharides Disaccharides Oligosaccharides polysaccharides Nomenclature Based on the no. of carbons with the suffix –ose added. CnH2On,n= no. of carbons Triose= three carbons Tetrose= four carbon Pentose = five carbon Hexose= six carbon cont. Nomenclature can also indicate the reactive groups. A) Aldoses are monosaccharides with aldehyde. B) ketoses are monosaccharides with a group containing a ketone. Sugar with aldehyde=aldohexoses. System of numbering the carbon . The carbon are numbered sequentially with aldehyde or ketone group being on the carbon with the lowest possible number. Cyclic forms of sugar Chemical and physical properties of many sugars have shown that cyclic forms predominate over open-chain structure, both in liquid and solid state. Cyclic form of sugar = pyranoses; they resemble pyran. Frutose= Furanose. The intermolecular hemiacetals.When an aldehyde reacts with alcohol. They are unstable compounds. Open chain and cyclic form Optical isomerism Asymmetric carbons: carbohydrates contain asymmetric carbons,those bounded to four different atoms or groups of atoms. Because of this asymmetry,carbohydrates are optically active and rotate the plane of polarized light. Optically active substances An optically active substance is one which can rotate the plane of polarisation of plane polarised light. if you shine a beam of polarised monochromatic light (light of only a single frequency - in other words a single colour) through a solution of an optically active substance, when the light emerges, its plane of polarisation is found to have rotated. The rotation may be either clockwise or anti-clockwise. Assuming the original plane of polarisation was vertical, you might get either of these results. Cont. If plane polarized light is rotated to the right (clockwise) ,the compound is dextrorotatory.(+). If plane polarized light is rotated to the left (counterclockwise) , the compound is called levorotatory. (-). configuration The simplest carbohydrate are the monosaccharides trioses for e.g glyceraldehydes,which has two optically active forms designated L and D form L- Glecarldehyde; D-Glyceraldehyde Cont. A sugar may be Dextrorotatory or levorotatory irrespective of its D or L configuration. Anomeric carbon : is a new asymmetric carbon that is created by cyclization at the carbon bound to oxygen in hemiacetal formation. If the hydroxyl on the anomeric carbon is below the plane of the ring ,it is said to be in the alpha position, if it is above the plane of the ring , it is said to be beta position Cont. Cont. In solution alpha and beta slowly change into an equilibrium mixture of both. This process is known as mutarotation. Glycosidic links Acetals : Hemiacetals reacts with alcohols to form acetals. Glycosides: a sugar react with an alcohols to form an acetal known as glycoside. If the sugar residue is glucose, the derivative is a glucoside, if fructose, it is fructoside , if galactose , it is a galactoside. When R is another sugar , the gylcoside is a disaccharaide. cont. Glycosidic linkages : Reading from left to right. Therefore , sucrose has an alpha 1,2 glycosidic linkage. The disaccharides maltose possess an unattached anomeric carbon atom, which may have either the alpha or beta configuration. The glycosidic linkage in maltose must be in the alpha 1,4 glycosidic configuration. maltose Polysaccharides Amylose is a linear unbranched polymer of alpha units in a repeating sequence of α 1,4 – glycosidic linkages. Amylopectin is a branched polymer of a α D glucose with α 1,4 glycosidic linkages and with α 1,6 branching points that occur at intervals of approximately 25 to 30 α D glucose residues. Glycogen is a major storage form of carbohydrates in animals found mostly in liver and muscles . It is highly branched form of amylopectin . Cont. Cellulose is composed of chain of d- glucose units joined by Beta 1,4 linkages. The chains are exclusively linear (i.e unbranched). 1. Cellulose is a structural polysaccharides of plants cells. 2.Although cellulose forms a part of the human diet(i.e diet in vegetables and fruits).it is not hydrolyzed by human enzymes systems. Carbohydrates derivatives A. Phosphoric acid esters of monosaccharides 1. Phosphorylation is the initial step in the metabolism of sugars. 2. phosphorylation sugars such as d – glucose 1phosphate are metabolic intermediates. Phosphorylation Cont. Amino sugars . In these a , hydroxyl group is replaced by an amino or an acetylamino group. D acetylglucosamine cont. 1. Glucosamine is the product of hydrolysis of chitin, the major polysaccharides of the shells of insects and crustaceans(arthopods). 2. Galactosamine is found in the polysaccharides of cartilage, chondroitin sulphate(oso3-). Cont. Sugars acid : are produced by oxidation of the aldehydic carbon , the hydroxyl carbon , or both. Ascorbic acid (vitamin C ) is a sugar acid. Deoxy sugars include 2- deoxyribose , found in DNA Sugar alcohols Monosaccharides , both aldoses and ketones may be reduced at the carbonyl carbon to the corresponding polyhydroxy alcohols(sugar alcohols). Aldoses yield the corresponding alcohols , while ketoses forms two alcohols because a new asymmetric carbon is found in the process. Cont. Commonly occuring aldoses and ketoses forms the following sugar alcohols: A. D-Glucose yields D sorbitol. B. D Mannose yields D mannitol. C. D Galactose yields dulcitol. D. D.Fructose,and ketose yields D mannitol and D sorbitol. Cont. There are enzyme sysytem in humans tissues that can reduce monosaccharides to their corresponding alcohols.e.g The formation of D-Fructose from D glucose via Dsorbitol in seminal vesicle. In cases of galactokinase deficency ,excess galactose may be reduced to dulcitol in the lens of the eye. Cont. Sugar alcohols are also normal constituents of cell components as for e.g ribitol which is the carbohydrates moiety of riboflavin, found in the electron carriers riboflavin phosphate and flavin – adenine dinucleotide.