Biochemistry PPT - Deer Park Independent School District

Deer Park High School North
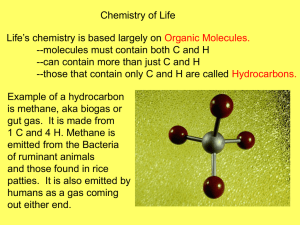
Biomolecules are organic molecules or carbon compounds because they contain carbon.
They are known as organic because the compose living things.
Organic = Living
Why is Carbon useful in forming living things?
Because:
Carbon forms STRONG bonds.
Carbon can form 4 bonds – continuous chainswhich can form large compounds. (macromolecules)
Carbon WANTS to bond with 4 other atoms.
There are 4 major organic molecules:
(biomolecules)
◦ Carbohydrates (sugars) – main energy source for all living things. (short energy storage)
◦ Proteins – make up the majority of our body; muscles, hair, skin, nails, etc. (Do NOT produce energy.)
◦ Lipids – Fats; Build up membranes. (Long term energy storage)
◦ Nucleic Acids – DNA which stores information and RNA
(“slave” to DNA) – major structures for hereditary information.
A MONOMER is a single organic molecule. These are the building blocks of biomolecules.
“MONO” means one; a single object.
When we start putting monomers together, we build a chain of monomers called a POLYMER.
“POLY” means many; many objects.
To create biomolecules, monomers must “link” together to form chains, which become polymers. These polymers have to be put together.
The process that builds monomers into polymers is called Dehydration Synthesis.
Dehydration
Synthesis
= removing water
= to put together
How do we pull polymers apart or break them down?
Add water back into the polymer to break apart the monomers back into single molecules.
The process used to break down large polymers into smaller monomers is called HYDROLYSIS.
Hydro -
Lysis -
To hydrate; add water
To break apart or digest
Functional Groups give functionality, behavior to the major organic molecules.
1.
There are 6 functional groups:
Hydroxyl
2.
3.
4.
5.
6.
Carboxyl
Amine
Methyl
Sulfudryl
Phosphate
Carbohydrates – Sugar molecules; Gives energy and structure
I.
The Structure of Carbohydrates
Made up of carbon, hydrogen and oxygen. The general formula is CH
2
O.
Typically, the formula for a sugar molecule is generally a 1:2:1 ratio.
Example: Common sugar, glucose, has the chemical formula of C
6
H
12
O
6
. In the chemical formula, the 6:12:6 ratio reduces to a 1:2:1 ratio.
II.
Functions of Carbohydrates
1.
Primary Energy Source (Quick Energy). Very little energy required to breakdown is the reason it’s used first. (Examples: Glucose, Sucrose, Fructose,
Galactose, Ribose, deoxyribose.)
2.
Energy Storage. [Examples: Starch (plants), Glycogen
(animals – muscle and liver)]
3.
Structural. [Examples: Celluose (plant fiber), Chitin
(exoskeleton, cell walls in fungi)]
** Many carbohydrate names end in ose.
III.
Production
Produced by green plants in chloroplasts undergoing Photosynthesis
IV.
Monomers (building blocks)
Monosaccharides – simple sugars
V.
Examples
1.
Monosaccharides (“Mono” = One, “Saccharide” = Sugar)
Glucose (C
6
H
12
O
6
) – Blood sugar, used for cellular respiration
Fructose – found in fruit
Galactose – found in milk
Deoxyribose – used in DNA (5 Carbon sugar – missing one
“O”)
Ribose – Used in RNA (5 Carbon Sugar)
Dehydration Synthesis joins two sugars together by removing an OH from one sugar and a H from the other to produce a new sugar and a water molecule.
2.
Disaccharides – (“ Di” = Two, “Saccharide” = Sugar)
Sucrose (C
12
H
22
O
11
) – Table Sugar (Glucose + Fructose)
Lactose – Sugar found in milk (glucose + galactose)
Maltose – Sugar found in germinating grain (glucose + glucose)
Let’s add one more sugar molecule to the disaccharide…Now we have 3 molecules put together by pulling water molecules out. We can do this over and over creating a polymer called a complex carbohydrate or POLYSACCHARIDE .
How do we undo what we just built? …Just reverse the process by adding water molecules back in. The bonds would need to be broken this time and OH and H are added back in during the process of HYDROLYSIS
(digestion).
Hydro (water) + Lysis (to cut) = cutting with water.
3.
Polysaccharides
– (“Poly” = many; “Saccharide” = Sugar)
If we add more sugar molecules to a disaccharide, then we will begin to have a long chain of sugar molecules that are bonded through Dehydration Synthesis. This creates a POLYMER of sugars which is a chain of many sugars that we call a COMPLEX
CARBOHYDRATE.
Starch (storage role) – bent chains: Hundreths of glucose molecules are attached over and over again.
Why are plants doing it?
Why do plants make these large molecules?
◦ To store excess sugars!
Can we (Animals) do that?
YES!
Glycogen (storage role) – Chains are branched
Thousands of glucose molecules are broken down into monosaccharides reattach them again and store a s glycogen in the liver.
We can get to that storage when we need it!
Chop up those monosaccharides in order to use them in our cells!
Cellulose (Structural role) – Straight chains
The structure of plants is made of cellulose.
Major component of the plants’ cell wall; commonly known as fiber (roughage).
They form straight chains, provide rigidity – gives the CRUNCH to fruits and vegetables!
There are hydrogen bonds between the polysaccharides, which make them very durable!
Chitin (Structural role) – Straight Linear Chains
Structure in the exoskeleton of arthropods and the cell walls of fungi.