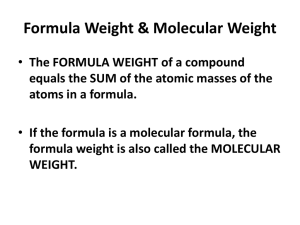
Quantitative Chem - Kent Science Resource Centre
advertisement

KENT TRIPLE SCIENCE NETWORK From Kent Science Resource Centre Formulae, Equations & Moles [Getting to grips with quantitative chemistry] How comfortable are you with quantitative chemistry? • • • Personal knowledge? Delivery to the students? Practical aspects? Difficulties and misconceptions • • • • • Students often lack secure understanding of basic chemical concepts. Many of the ideas involved are abstract and/or difficult to comprehend: • ‘amount of substance’ (or ‘chemical amount’); • Avogadro’s number (too large to be easily ‘seen’); • link between macroscopic and microscopic scales. The definition of ‘mole’ often seems quite confused. The language & vocabulary used can cause problems. The ‘mole’ is often taught purely as an abstract mathematical idea. Important basic concepts …(1) • Matter is made from tiny particles (atoms) invisible to the naked eye. • Chemical reactions produce new substances. • In a chemical reaction, atoms are rearranged but no atoms are created or destroyed. Mass is conserved. • Chemists need to be able to accurately measure amounts of substance in order to be able to control/manipulate chemical reactions. Conservation of mass (1) Conservation of mass (2) Conservation of mass (3) Sealable bag with citric acid and sodium bicarbonate. Weighed – with water – before and after, Important basic concepts …(2) • Chemical formulae – understanding and using. • Relative atomic mass (Ar). • Relative molecular (or formula) mass (Mr). • Balanced symbol equations – interpreting/writing. Understanding chemical formulae [Important basic concepts] Understanding chemical formulae [Important basic concepts] 2− 3+ 2+ 3− 1− 1+ Understanding chemical formulae [Important basic concepts] Advantages/disadvantages of this model? 1− 2+ 1− Web simulations Two from PhET Build a molecule http://phet.colorado.edu/en/simulation/build-a-molecule Balancing Chemical equations http://phet.colorado.edu/en/simulation/balancing-chemicalequations Understanding chemical formulae [Important basic concepts] H2O Advantages/disadvantages of different models? Relative atomic mass [Important basic concepts] 100 chlorine atoms 75 atoms are 35Cl 25 atoms are 37Cl Total mass of all 100 atoms = (75 x 35) + (25 x 37) = 3550 So … the average mass of a chlorine atom is 3550/100 = 35.5 i.e. Ar(Cl) = 35.5 Relative molecular (formula) mass [Important basic concepts] One model that can help some students ‘see’ that relative molecular mass (Mr) = sum of the Ar values of the component elements. Mr(H2O) = 1+ 1+ 16 = 18 Balanced equations [Important basic concepts] 2H2 + O2 2H2O Link to a real reaction Mix different ratios of methane and oxygen in 500ml plastic bottles. Which gives the most violent reaction. Why are some reactions more explosive? Which ratio gives the loudest explosion? Why? Follow CLEAPSS risk assessment. Wear ear plugs! Reacting Masses Experiment The change in mass when magnesium burns http://www.youtube.com/watch?v= d8QWXCaXfSs Analysing results 1. Record class results in a spreadsheet and produce a line of best fit. • Show that there is a fixed ratio of Mg:O 2. Plot individual results onto a pre-prepared graph • Identify the most likely formula 3. Wait until mole has been covered and calculate empirical formula • Calculate the formula MgO Experiment Analysing Epsom salts A weighed sample of Epsom salt (magnesium sulphate) is heated in a crucible to drive off the water of crystallization. Simple graphs could show that there is a fixed ratio of salt to water. Later, (using moles) the formula of the salt could be determined. Introducing the ‘mole’ Introducing ‘the mole’ Thinking it through … Take two elements: Cu S (Ar = 64) (Ar = 32) Introducing ‘the mole’ Thinking it through … Take two elements: What would be the ratio of the relative masses if you had • 1 atom ofQ. each element? Is it possible to weigh one atom in our lab? Cu S (Ar = 64) 2 (Ar = 32) : 1 Introducing ‘the mole’ Thinking it through … Take two elements: What would be the ratio of the relative masses if you had • 1 atom of each element? • 100 atoms of each element? • 1000 atoms of each element? • 1 million atoms of each element? Cu S (Ar = 64) 2 : (Ar = 32) 1 Introducing ‘the mole’ Thinking it through … Q. What is the ratio of the actual masses of the two elements? Q. What can you say about the numbers of atoms in the two samples? How do they compare? 64 g of Cu 32 g of S Avogadro’s number • • • • • In the samples of Cu and S: the atoms are very small the number of atoms present is very large the number of atoms present is called Avogadro’s number (6.02 x 1023) we refer to this number and the physical amount of substance (measured in g) as ‘one mole’ See the ‘Astounding numbers’ activity from Chemistry for the Gifted and Talented (Tim Jolliff, RSC 2007). Magic Triangles Working out the relationship Q. What is the mathematical relationship between moles, Mr and mass? Reacting quantities Calculate the yield of copper sulphate – worksheet 13. Moles & Gases Avogadro’s Law (hypothesis/theory Equal volumes of gases at the same temperature and pressure contain the same number of molecules. Turning this around: Equal numbers of molecules (measured under the same conditions) will occupy the same volume So, Avogadro’s number of molecules (a mole) of any gas will occupy the same volume. At room temperature and pressure this volume is 24dm3. Experiment Can we estimate the Avogadro volume? A quick and easy method using a known mass of zinc carbonate to produce carbon dioxide, the volume of which is measured in a gas syringe Experiment The volume of 1 mole of hydrogen gas A method using magnesium and hydrochloric acid and simple apparatus found in most labs. Celebrate the Mole National Mole Day is October 23rd. http://www.moleday.org/ Celebrated from 6.02 am to 6.02 pm Thus 6.02 10/23 How could you celebrate it? Additional resources and links http://www.ulster.ac.uk/scienceinsociety/molepack.html Mole Day Pack http://www.rsc.org/images/Misconceptions_update_tcm18188603.pdf ‘Beyond appearances’ http://www.rsc.org/Education/Teachers/Resources/Books/Misco nceptions.asp Selected activities from RSC Misconceptions http://www.rsc.org/Education/Teachers/Resources/Aflchem/ Assessment for Learning Chemistry http://www.mindsetsonline.co.uk/product_info.php?products Mindsets (for the Ionic Jigsaws) _id=1141 http://www.cleapss.org.uk/ CLEAPSS website link to their homepage