SI Units and Gas Laws
advertisement

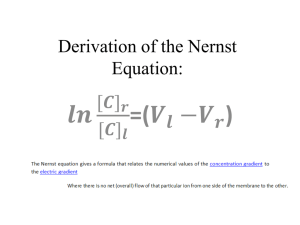
Anesthesia Department S.I. units Gases and Gas Laws By Ahmed Ibrahim ; M.D. Prof.of Anesthesia Ain Shams University Intended Learning Outcomes By the end of this lecture , the student will be able to: 1..know different units used for clinical measurements . 2..understand different laws controlling ideal gas behavior. 3..interpret the applications of different gas laws in anesthetic practice. © Basic 7 S.I units Meter m length Kilogram Kg mass Second s time Ampere A electric current Kelvin oK temperature Mole mol amount of substance Candela cd luminous intensity Fractions & Multiples 10-18, 10-15, 10-12, 10-9, 10-6, 10-3, 10-2, 10-1 10 102 103 106 109 1012 1015 1018 atto femto pico nano micro milli centi deci deca hecto kilo mega giga tera penta exa a f p n µ m c d Da h K M G T P E •area m2 •volume m3 •velocity displacement / time ms-1 •acceleration velocity / time ms-2 •force mass x acceleration Kgms-2 •work (energy) force x distance Kgm2s-2, •power work / time Kgm2s-3, •pressure force / area •frequency •electric charge Newton N Nm Joule J Js-1 Watt W Nm-2 Pascal Pa cycles / second Hertz electric current x time Coulomb Hz C Volt V Ohm Ω •electric capacitance Farad F •magnetic flux Weber Wb •potential difference (EMF) •electric resistance potential difference / current SOLID LIQUID GAS/VAPOUR +++ + ± ++ ++ ++ + +++ +++ SHAPE OF CONTAINER NO YES YES MISSIBILITY NO YES YES I.M.FORCE I.M.SPACE MOLECULAR MOTION VAPOUR gaseous state of the substance which is present in the liquid form at RTP GAS a substance which cannot exist in the liquid form at RTP Kinetic Theory of Gases GAS MOLECULES are: •Widely separated (negligible molecular volume) •In constant motion (diffusion) •In constant striking (pressure) IDEAL (PERFECT) GAS, obeys the 3 assumptions VOLUME GAS TEMP PRESSURE Robert Boyle (1627-1691). , Ireland Jacques Charles (1746-1823) Critical Temperature “ the temperature above which a gas cannot be liquefied whatever pressure is applied ” Critical Pressure ” the pressure needed to liquefy a gas at its critical temperature “ N.B. in T in P needed CO2 N2O O2 31oC 36.5 oC -116oC (C.P = 74 atm) Joseph Louis Gay-Lussac (1778-1850) for a given mass of a gas, at a constant: TEMPERATURE PRESSURE VOLUME V T(oA) V 1/P P x V =Const. V=T x Const. (K) P1xV1= P2xV2 V/T = K Boyle’s law Charles' law P T Gay-Lussac’s law a Perfect Gas ,is the one that always obey Boyle’s and Charles’ laws. in which the intermolecular forces and molecular volume are too small ( negligible ). a Real Gas behaves approximately as a perfect gas especially at low pressures and high temperatures. Equation of state of a perfect gas: V 1/P V V T/P V = T/P x const PV/T = const (R) P1V1 / T1 = P2V2 / T2 T Avogadro’s hypothesis Amadeo Avogadro (1776-1856) Italy for perfect gases at EQUAL 1 mole of any gas at , Volumes Temp Pressure STP Room temp Body temp contain equal number of molecules 22.4 L 24.1 L 25.4 L NB. a mole of volatile liquid (e.g. 197.4 gm Halothane) PV=RT 22.4 L vapour at STP R (molar gas constant) = 1 x 22.4 / 273=0.082 litre.atm /degree oA/ mole =1.987 joule / degree / mole (S.I) A mole of any gas contains the same number of molecules = 6.02 x 1023………… ……………….” Avogadro’s number” John Dalton 1766-1844 DALTON’S law of Partial Pressure “ the pressure exerted by a mixture of gases (and/ or vapours) – enclosed in a given space – is equal to the sum of pressures which each gas (or vapour) would exert if it alone occupied the same space” Partial Pressure (P.P) = total pressure x volume % Volume % = (P.P / total P ) x 100 Saturated Vapor Pressure (SVP) •nature of liquid •its temperature mmHg (20oC) -Desflurane 670 -Diethyl ether 440 -Halothane 243 -Isoflurane 239 -Enflurane 175 -Sevoflurane 160 -Trilene 60 -Methoxyflurane 23 varies as a function of: Summary of important points •Basic S.I units - length = Meter m - mass = Kilogram Kg - time = Second s - electric current = Ampere A - temperature = Kelvin oK - amount of substance = Mole mol - luminous intensity = Candela cd •Derived units - area = m2 - volume = m3 - velocity =ms-1 - acceleration = ms-2 - force =Kgms-2 = Newton N - work (energy) = Kgm2s-2 = Nm = Joule J - power = Kgm2s-3 = Js-1 = Watt W - pressure = Nm-2 = Pascal Pa - frequency = Hertz Hz - electric charge = Coulomb C - potential difference (EMF) =Volt V - electric resistance = Ohm Ω - electric capacitance = Farad F - magnetic flux = Weber Wb •VAPOUR is the gaseous state of the substance which is present in the liquid form at RTP •GAS is a substance which cannot exist in the liquid form at RTP •Boyle’s law : V 1/P at constant temperature •Charles' law : V T (oA) at constant pressure •Gay-Lussac’s law : P T at constant volume •Critical Temperature : “ the temperature above which a gas cannot be liquefied whatever pressure is applied ” •Avogadro’s hypothesis : “EQUAL volumes of perfect gases at same temperature and pressure contain same number of molecules” •Avogadro’s number : A mole of any gas contains the same number of molecules = 6.02 x 1023 •DALTON’S law of Partial Pressure Partial Pressure (P.P) = total pressure x volume % Examples of questions to assess the ILOs 1. Which of the following units are BASIC SI units of measurement? a) Kilometre. b) Candela. c) Watt. d) Kilogram. 2.The following are correct S.I. Units a) The unit of energy is the Newton b) The unit of power is the Watt c) The unit of frequency is the Hertz d) The unit of mass is the gram a) The unit of length is the metre 3.The critical temperature is: a) 273 K b) the temperature above which a gas cannot be liquified by pressure alone c) the temperature below which a gas does not vaporise d) 36.5 degrees C for nitrous oxide e) different if a substance is in a mixture rather than on its own 4.Concerning the gas laws a) Boyle's law refers to the relationship between temperature and pressure of a gas b) Temperature is measured on the absolute temperature scale c) Temperature is a constant in Charles' law d) Boyle's law states that at a constant volume pressure varies with temperature e) The gas laws are only true for air 5.One mole of a gas a) Occupies 22.4 Litres at room temperature b) Has the same volume for any gas c) Contains Avogadro's number of molecules d) May be liquefied by compression if above critical temperature e) Is one gram molecular weight Answers 1. false, true, false, true 2. false, true ,true, false, true 3. false, true, false, true, true 4. false, true, false, false, false 5. false, true, true, false, true Thank You