Chem Comm Unit1
advertisement

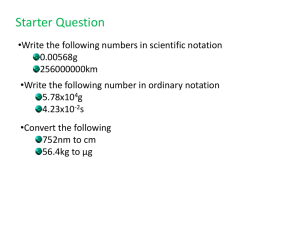
UNIT 1: WATER: EXPLORING SOLUTIONS MISS SHUEY CHEM COMM ESSENTIAL QUESTIONS • What techniques can we use to purify water? • What are the physical properties of water? • Why do some substances readily dissolve in water and others do not? • How does chemistry contribute to effective water treatment? ARTICLE REVIEW • Fish Kill Triggers Riverwood Water Emergency SECTION A: SOURCES AND USES OF WATER • Uses of water: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. _________________________ _________________________ _________________________ _________________________ _________________________ _________________________ _________________________ _________________________ _________________________ _________________________ A.4 WATER SUPPLY AND DEMAND • Family of four uses 390 gallons daily. • Direct water use – volume that can be directly measured. • Indirect water use – hidden uses of water that you may never have considered. • Ex. Slice of pizza?? Figure 1.12 A5. WATER USE IN THE US • For each region in the US, name the greatest single use of water. • A. the east b. the south c. the midwest • D. the west e. alaska f. hawaii Explain the differences in how water is used in the east and the west. Think about where most people live and where most of the nation’s factories and farms are located. What other regional factors help explain the general patterns of water use? List two factors about the weather, economy, or culture that could explain the greatest water use within each of these six U.S. regions. A.6 WHERE IS THE WORLD’S WATER? • 97% of the world’s water. 1. Ocean 2. Glaciers PHYSICAL STATES OF WATER • Gaseous state: water vapor • Liquid state: lakes, rivers, oceans, clouds, and rain. • Solid state: ice CITY WATER • Surface water: water supply originated in a river or other body of water. • Ground water: water in a well. RURAL WATER • Aquifer: water-bearing layer of rock, sand, or gravel, then pumped to the surface. A.8 RIVERWOOD WATER USE • Pg.22 • http://www.youtube.com/watch?v=xxNfJLMNS4E&li st=FLHywkjQDas46hk_l2QAeKaA&feature=mh_lolz SCIENTIFIC METHOD Hypothesis – testable statement controls – remain constant variable – is changed Model – explanation of how phenomena occur and how data or events are related. Theory – broad generalization that explains a body of facts or phenomena. ACCURACY AND PRECISION • Accuracy – close to the expected value • Precision – a number of measurements close to each other. What is more accurate? Graduated cylinder Or Beaker SIGNIFICANT FIGURES • Indicates how precise a measurement is. rule example 1. Zeros between other nonzero a. 50.3 m has three sig figs digits are significant b. B. 3.0025 s has five sig figs 2. Zeros in front of nonzero digits are not significant. a. 0.892 kg has three sig figs b. 0.0008 ms has one sig fig 3. Zeros that are at the end of a number and also to the right of the decimal are significant a. 57.00 g has four sig figs b. B. 2.000000 kg has seven sig figs 4. Zeros at the end of a number but to the left of a decimal are significant if they have been measured or are the first estimated digit; if not they are NOT significant. a. 1000 m may contain from one to four sig figs, depending on the precision of the measurement, in this book it will be assumed there is one sig fig. b. 20 m has one sig fig (scientific notation will indicated sig fig number) RULES FOR CALCULATING WITH SIG FIGS Type of calculation Rule example Addition or subtraction When measurements are added or subtracted, the answer can contain no more decimal places than the least accurate measurement 97.3 + 5.85 --------103.15 103.2 Round off Multiplication or division The final answer has the 123 same number of sig figs x 5.35 as the measurement ------------658.05 658 having the smallest number of sig figs. Round off SIG FIG PRACTICE Perform these calculations following the rules for sig figs. a. 26 x 0.02584 = ? b. 15.3 / 1.1 = ? c. 782.45 - 3.5328 = ? d. 63.258 + 734.2 = ? SI unit – measurements in science. Volume Density – m/v A sample of aluminum metal has a mass of 8.4g. The volume of the sample is 3.1 cm3. calculate the density of aluminum. Dimensional analysis – math technique that allows you to use units to solve problems involving measurements. SECTION B • Article reading • B.1 PHYSICAL PROPERTIES OF WATER • Matter – anything that occupies space and has mass. • Physical properties: observed and measured without changing the substance. • Density – mass of material within a given volume. • D = m/v WATER PROPERTIES • Freezing point – 0 C when liquid water forms a solid. • Aqueous solution – water-based solution. • Important aqueous solutions in your life: • ____________________ • ____________________ • ____________________ B.3 MIXTURES AND SOLUTIONS • Mixture – when two or more substances combine and retain their individual properties. • Heterogeneous (suspension) • Homogeneous (solution) • Solute • Solvent • Pg.30 B.4 PARTICULATE VIEW OF WATER • Particulate level – at the level of its atoms and molecules. • Atoms – building blocks of matter. • Element – matter made up of only one type of atom. • Compound – composed of the atoms of two or more elements bonded together in fixed proportions. • Ex. • Chemical formulas – representing compounds or elements, showing ratios of how they bond. • Ex. • Substance – element or compound with uniform and definite compositions. • Molecule – smallest unit of a molecular compound that retains the properties of that substance. B.5 PICTURES IN THE MIND • Macroscopic – large-scale, easily observed without microscopes or other tools. • Models – representations of atoms and molecules. • Pg.33 ques. 1-7 B.6 SYMBOLS, FORMULAS, AND EQUATIONS • Chemical symbols – letters to represent element. • Periodic table of the elements – arrangement of elements according to the number of protons. COMMON ELEMENTS Aluminum Al Bromine Br Calcium Ca Carbon C Chlorine Cl Cobalt Co Copper Cu Fluorine F Gold Au Phosphorus P Silver Ag Sulfur S Hydrogen Iodine Iron Lead Magnesium Mercury Nickel Nitrogen Oxygen Potassium Sodium Tin H I Fe Pb Mg Hg Ni N O K Na Sn WHAT ELEMENT IS THIS? K potassium WHAT ELEMENT IS THIS? Al aluminum WHAT ELEMENT IS THIS? Br bromine WHAT ELEMENT IS THIS? Ca calcium WHAT ELEMENT IS THIS? C carbon WHAT ELEMENT IS THIS? Cl Chlorine WHAT ELEMENT IS THIS? Co Cobalt WHAT ELEMENT IS THIS? F Fluorine WHAT ELEMENT IS THIS? Au Gold WHAT ELEMENT IS THIS? H Hydrogen WHAT ELEMENT IS THIS? I Iodine WHAT ELEMENT IS THIS? Fe Iron WHAT ELEMENT IS THIS? Pb Lead WHAT ELEMENT IS THIS? Mg Magnesium WHAT ELEMENT IS THIS? Hg Mercury WHAT ELEMENT IS THIS? Ni Nickel WHAT ELEMENT IS THIS? N Nitrogen WHAT ELEMENT IS THIS? O Oxygen WHAT ELEMENT IS THIS? P Phosphorus WHAT ELEMENT IS THIS? Ag Silver WHAT ELEMENT IS THIS? Na Sodium WHAT ELEMENT IS THIS? S Sulfur WHAT ELEMENT IS THIS? Sn Tin WHAT IS THE ATOMIC NUMBER? O – Oxygen 8 WHAT IS THE ATOMIC NUMBER? C – Carbon 6 WHAT IS THE ATOMIC NUMBER? H– Hydrogen 1 WHAT IS THE ATOMIC NUMBER? Au – Gold 79 WHAT IS THE ATOMIC NUMBER? N – Nitrogen 7 B.8 ELECTRICAL NATURE OF MATTER • Electrons – negatively charged particles • Protons – positively charged particles, in nucleus • Neutrons – neutral particles, in nucleus B.9 IONS AND IONIC COMPOUNDS • Ions – electrically charged atoms or groups of atoms. • Ionic compounds – substances composed of positive and negative ions • Cation – positive ion Na+ • Anion – negative ion Cl• Polyatomic ion – ion consisting of a group of bonded atoms B.11 WATER TESTING • Precipitate – insoluble material in water. • Qualitative test – looking at non numerical descriptions. • Quantitative tests – numerical data. • Ionic compounds • Metal + nonmetal B.12 PURE AND IMPURE WATER • Pg. 45 • Gases in atmosphere dissolve in water, nitrogen, oxygen, carbon dioxide • 46-47 Read SEC. C INVESTIGATING THE CAUSE OF THE FISH KILL • Solubility – substance that will dissolve in water. • Saturated – maximum quantity of a substance that will dissolve in a quantity of water. • What effects solubility? • Solubility curve – relationship between temperature and solubility • Unsaturated solution – solution that contains less dissolved solute than the amount that the solvent can normally hold at that temperature. • Supersaturated solution – unstable solution that contains more solute than could usually be dissolved at that temperature. • Ex. Rock candy (pg.55) C.4 DISSOLVING IONIC COMPOUNDS • Form ions in water • Polar molecule – electrons are not evenly distributed throughout its structure. • Partial positive region and partial negative region. • Ex. water DIATOMICS Reactant and Products are shown in chemical equations Reactant are the substances that are used to make a chemical reaction Products are substances produced by the reaction Na + Cl ---> NaCl reactant --> products Diatomic Molecules - some elements bond to themselves, like H2 GEN-U-INE diatomic molecules U should remember that the diatomic molecules all end in -GEN or -INE (or H N F O I C B) H2 N2 F2 O2 I2 Cl2 Br2 C.8 INAPPROPRIATE HEAVY-METAL ION CONCENTRATIONS? • Essential Metal Ions: • • • • Iron(II) Fe2+ Potassium K+ Calcium Ca2+ Magnesium Mg2+ Heavy-metal ions – atoms have greater masses than those of essential metallic elements, harmful to humans and other organisms. • Lead Pb2+ -bind to proteins in biological systems, • Hg2+ prevents proteins from performing their normal task. HEAVY METAL HARM • Damage to the nervous system, brain, kidneys, and liver, which can even led to death. • They become concentrated within the bodies of fish and shellfish. • Costly to remove • Hard to detect • Prevention: using alternate materials in industry. Called Green Chemistry LEAD IONS (PB2+) • Latin name – plumbum • Plumber – water pipes in ancient Rome were commonly made of lead. • Used in: • Pottery, automobile electrical storage batteries, solder, cooking vessels, pesticides, and paints Many were replaced with iron, copper or plastic materials. 1970s lead was added to gas to produce a better-burning automobile fuel. Released in atmosphere. MERCURY IONS HG2+ • Liquid at room temp • Latin name = hydrargyrum, quicksilver or liquid silver • Uses: • Electrical conductor, thermometers, thermostats, hats, light bulbs, pesticides • Vapor is hazardous, absorbed through skin CHEMICAL POSTER C.9 INAPPROPRIATE PH LEVELS • pH – measure and report the acidic, basic, or chemically neutral character of a solution. • Range = 0---14 • Neutral = 7 7> acidic 7<basic (alkaline solutions) 1pH unit = tenfold difference in acidity or alkalinity pH 3 soft drinks, pH 2 lemon juice Lemon juice is 10 time more acidic than soft drinks. LITMUS PAPER • • • • • • • • Indicator to show level of acidity or alkalinity. Blue – basic Red – acidic Acidic and basic solutions conduct electricity. What does this tell you? Ions present in the solution. Acids – release H+ ions Bases – release OH- ions NEUTRAL SUBSTANCES • Sucrose, sodium chloride solutions = neutral • Low pH in streams • Fish-egg development is impaired • Increase the concentrations of metal ions by leaching metal ions from surrounding soil. High pH in streams • alkaline solutions are able to dissolve organic materials, including skin and scales EPA REQUIREMENTS • Drinking water be within the pH range of 6.5 – 8.5 • Fish can tolerate 5.0 – 9.0 • Did the pH change to kill all the fish? C.10 INAPPROPRIATE MOLECULAR SUBSTANCE CONCENTRATIONS • Molecular substances – composed of molecules not ions. • Molecular substances can be harmful for aquatic life. • Examples: ethanol C2H5OH, succinic acid C4H6O4, carbon dioxide CO2, oxygen gas O2 • What determines the solubility of a molecular substance in water? • Distribution of electrical charge within molecules. • Electronegativity – ability of an element’s atoms to attract shared electrons when bonding within a compound. causes e- to be unevenly distributed among the atoms. POLAR MOLECULE • • • • Negative and positive side of a molecule. “like dissolves like” Polar dissolves polar Nonpolar dissolves nonpolar • Ex. Oils, soaps soap attracts oils C.11 SOLVENTS • Soluble – will dissolve • Insoluble – will not dissolve C.12 INAPPROPRIATE DISSOLVED OXYGEN LEVELS? • As temperature goes up less oxygen is dissolved. • Gas solubility in water is directly proportional to the pressure of that gaseous substance on the liquid. • Increase in water temperature affects fish by decreasing the amount of dissolved oxygen in the water and by increasing the oxygen consumption of fish.