Significant Figures
advertisement

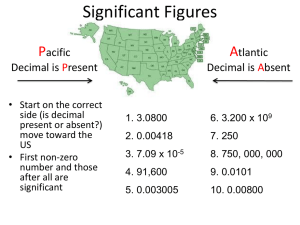
Significant Figures Unit 1 Presentation 3 Scientific Notation The number of atoms in 12 g of carbon: 602,200,000,000,000,000,000,000 6.022 x 1023 The mass of a single carbon atom in grams: 0.0000000000000000000000199 1.99 x 10-23 N x 10n N is a number between 1 and 10 n is a positive or negative integer Scientific Notation 568.762 0.00000772 move decimal left move decimal right n>0 n<0 568.762 = 5.68762 x 102 0.00000772 = 7.72 x 10-6 Addition or Subtraction 1. Write each quantity with the same exponent n 2. Combine N1 and N2 3. The exponent, n, remains the same 4.31 x 104 + 3.9 x 103 = Scientific Notation Multiplication 1. Multiply N1 and N2 2. Add exponents n1 and n2 Division 1. Divide N1 and N2 2. Subtract exponents n1 and n2 (4.0 x 10-5) x (7.0 x 103) = 8.5 x 104 ÷ 5.0 x 109 = Significant figures (sig figs) How many numbers in a measurement mean something When we measure something, we can (and do) always estimate between the smallest marks. 1 2 3 4 5 Significant figures (sig figs) The more marks the better we can estimate. Scientists understand that the last number measured is actually an estimate 1 2 3 4 5 Sig Figs What is the smallest mark on the ruler that measures 142.15 cm? 142 cm? 140 cm? Here there’s a problem: Does the zero count or not? Scientists needed a set of rules to decide which zeroes count. All other numbers always count Which zeros count? Leading zeros never count – 0.045 Trapped zeros always count – 100365405.057 Trailing zeros only count if there is a decimal place present – 12400 – 12400. Here the zeroes do NOT count Here the zeroes DO count Sig Figs Only measurements have sig figs. Counted numbers are always exact – A dozen is exactly 12 A a piece of paper is measured 11 inches tall. Being able to locate, and count significant figures is an important skill. Sig figs. Count the sig figs and the number of significant zeros in the following numbers – – – – – – 458 g 4085 g 4850 g 0.0485 g 0.004085 g 40.004085 g Adding and subtracting with significant figures The last significant figure in a measurement is an estimate. Your answer can not be better (more precise) than your worst (least-precise) estimate. You have to round it to the least place of precision of the measurement in the problem For example 27.93 + 6.4 + First line up the decimal places Then do the adding 27.93 Find the estimated 6.4 numbers in the problem 34.33 This answer must be rounded to the tenths place Practice 4.8 + 6.8765 520 + 94.98 0.0045 + 2.113 6.0 x 102 - 3.8 x 103 5.4 - 3.28 6.7 - .542 500 -126 6.0 x 10-2 - 3.8 x 10-3 Multiplication and Division Rule is simpler Answer will have the same number of sig figs as the value with the least number of sig figs in the problem 3.6 x 653 = 2350.8 3.6 has 2 s.f. 653 has 3 s.f. answer can only have 2 s.f. 2400 Note that there is NO decimal point present! Multiplication and Division Same rules for division Lets do some practice. 4.5 / 6.245 4.5 x 6.245 9.8764 x .043 3.876 / 1983 16547 / 714