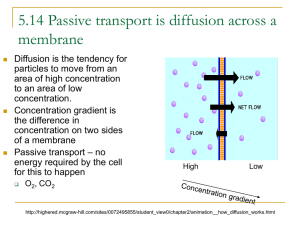
Gummi Bear Lesson for Sept 16 - Colorado Springs School District 11
advertisement

Welcome For the next 45-55 minutes we are asking you to engage in the activity as a learner. After the lesson we will look at the lesson through your eyes - the eyes of a teacher. Open up your science notebook… Begin a new entry, possible title: “Concentration and Osmosis” Include today’s date. Record the focus question for today’s lesson. Focus Question: How is the concentration of a solution related to its effects on other substances? Respond to the following… Drinking water in our homes contains low concentrations of dissolved chlorine, a highly toxic substance. Why is it safe to drink the water? What do you think ‘concentration’ means? Where have you seen this term before? When you are done writing, turn to a neighbor and share your thinking. Feel free to write down additional information. Now take a look at this… tp://www.youtube.com/watch?v=H6N1IiJTmnc Record your observations in your science notebook. Then explain why this video is called “osmosis in the kitchen”. What is osmosis? Where have you heard this term before? When you are done writing, turn to a neighbor and share your thinking. Feel free to write down additional information. Consider this… More than 36 million people in North America use contact solution to store their contacts. Most contact lenses solutions contain some saline solution (water that contains dissolved salts.) One purpose of the saline solution is to keep the water balance of the lenses correct. Why do you think this ‘water balance’ is important? What would you recommend to someone that did not have any contact solution to store their contacts in overnight? When you are done writing, turn to a neighbor and share your thinking. Feel free to write down additional information. Today we will explore some of these questions by examining gummi bears… History of Gummi Bears • The gummi bear originated in Germany • Haribo company 1922, the Dancing Bear, a fruitflavored gum made in the shape of a bear - later become Haribo's world-famous Gold-Bears candy product in 1967. • Trolli (another gummi candy manufacturer) first introduced gummi worms in 1981. • Gummi bears are also one of the few types of candy to have been turned into a television show. Gummi Bears - Typical Ingredients •sugar •glucose syrup •starch •flavoring •food coloring •citric acid •gelatin Gummi Bears Gummy Bears • 1 gummy bear in each cup • Soaked overnight in… (2-3 hr minimum) – water – 0.1 M salt soln, 0.5 M salt soln, 1.0 M salt soln – 0.1 M sugar soln, 1.0 M sugar soln, 2.0 M sugar soln – corn syrup – air • What do you think will happen? Record your thoughts in your science notebook. Be specific. Background Information A solution is a mixture of two or more substances that is uniform throughout. The substance that is present in the greatest amount is called the solvent, the solute is dissolved in the solvent. Background Information Concentration is the amount of solute present in a mixture or solution for a specified amount of solvent. A common measure of concentration is moles per L, moles/L, which is referred to as molarity, M. When a substance is diluted it means it is less concentrated. Gummi Bears Gummy Bears In your science notebook, record your scientific* observations of the gummy bears. * thorough * accurate * qualitative * quantitative * * When you are done, rank the gummy bears according to size from smallest to largest. Record your ranking. Gummi Bears Gummy Bears When you are finished making observations, please clean up ! • Remove gummy bears from solutions, place them in a paper towel and place the towel in the trash. • Pour the solutions down sink, rinse the cups and spoons with water, and dry them all before returning them to the ziplock bag provided. – If you mix some water with the corn syrup, it will come out of the cup easier. Discuss these questions with your group and record your best thinking: 1. What does it mean when a scientist says that a 1.0 M solution is more concentrated than a 0.1 M solution? 2. How does the size of the gummy bear relate to the concentration of the solution it is in? 3. How do the sizes of the gummy bears in the sugar solutions compare with those in the salt solutions? 4. What is happening – at the particle* level – to explain your observations? Be sure to include a diagram that shows the movement of particles into or out of the bears. (*particles refer to molecules and ions) You may want to refer to the word wall to incorporate some scientific vocabulary into your explanations. Word Wall • • • • • Observations Qualitative Quantitative Claim Evidence • • • • • • • Solution Solute Solvent Particles Concentration Molarity Diluted • Diffusion • Osmosis • Selectively permeable • Passive transport • Active transport Discuss one or more of these questions with the class. Be sure to record any changes in your thinking because of the class discussion. 1. What does it mean when a scientist says that a 1.0 M solution is more concentrated than a 0.1 M solution? 2. How does the size of the gummy bear relate to the concentration of the solution it is in? 3. How do the sizes of the gummy bears in the sugar solutions compare with those in the salt solutions? 4. What is happening – at the particle* level – to explain your observations? Be sure to include a diagram that shows the movement of particles into or out of the bears. (*particles refers to molecules and ions) Read the handout provided by your teacher. Compare the information in the handout to your best thinking about the questions below. Be sure to record any changes in your thinking because of what you read. 1. How does the size of the gummy bear relate to the concentration of the solution it is in? 2. How do the sizes of the gummy bears in the sugar solutions compare with those in the salt solutions? 3. What is happening – at the particle* level – to explain your observations? (*particles refers to molecules and ions) Handout: Explaining the Gummy Bear Observations In the gummy bears, the sugar could not move into and out of the bears easily – probably because of the presence of the gel. Instead, it is the water moving from an area of high concentration – of water – to an area of lower concentration – of water. The gummy bears plumped up the most when they were placed in water. This is because the concentration of water outside the bear was much higher than the concentration of the water inside the bear. The water will continue to move until the concentrations of substances inside and outside of the bear are the same. When the bears were placed in a solution with a high concentration of sugar, the bears only plumped up a little. When the bears were placed in a solution with a concentration of sugar higher than that of the bear, the bear became smaller. In the case of the corn syrup, the concentration of water inside the bear was actually higher than the concentration of water outside of the bear at the beginning of the experiment. Dots represent sugar molecules. Arrows show the direction of water movement. Handout: Osmosis / Diffusion • Osmosis is the movement of water across a selectively permeable membrane from an area of high water potential (low solute concentration) to an area of low water potential (high solute concentration). • Osmosis is a passive process, like diffusion which describes the spread of particles through random motion from regions of higher concentration to regions of lower concentration. • Net movement of solvent is from the less-concentrated (hypotonic) to the more-concentrated (hypertonic) solution, which tends to reduce the difference in concentrations. • The osmotic pressure is defined to be the pressure required to maintain an equilibrium, with no net movement of solvent. Osmotic pressure is a colligative property, meaning that the osmotic pressure depends on the molar concentration of the solute but not on its identity. Take a look back… Consider the opening questions to this lesson… chlorine in drinking water improving wilted lettuce or contact solution What have you learned today that would change your response to the question(s)? Record these thoughts. Exit Ticket – Today’s purpose: Explain how the concentration of a solution is related to its effects on other substances? Write your response to the question below on an index card. Be sure to put your name, date and period on the card. Turn the card in to your teacher before you leave class. What would happen if you placed a gummy bear in a 1.5 M sugar solution overnight? Use your data to help determine the outcome. Draw a picture showing which way the molecules are moving. Explain your answer in terms of the diffusion of water in or out of the bear. The lesson is completed at this point. Now we are going to ask you to consider the lesson as a teacher. Discussion question 1: How does the lesson you just participated in support the new Colorado Science Standards? Work with 1 or 2 other people to highlight where you see this lesson aligning with the standards. Be sure to consider both the left and the right side of the standards page(s). • handout of HS and MS standards provided Record and discuss your findings. Colorado Science Standards Science Grade Level Expectations at a Glance Standard Grade Level Expectation High School 2. Life Science 3. Cellular metabolic activities are carried out by biomolecules produced by organisms. 4. The energy for life primarily derives from the interrelated processes of photosynthesis and cellular respiration. Photosynthesis transforms the sun’s light energy into the chemical energy of molecular bonds. Cellular respiration allows cells to utilize chemical energy when these bonds are broken. 5. Cells use the passive and active transport of substances across membranes to maintain relatively stable intracellular environments. 6. Cells, tissues, organs, and organ systems maintain relatively stable internal environments, even in the face of changing external environments. Content Area: Science Standard: 2. Life Science Prepared Graduates: • Analyze the relationship between structure and function in living systems at a variety of organizational levels, and recognize living systems’ dependence on natural selection Grade Level Expectation: High School Concepts and skills students master: 5. Cells use passive and active transport of substances across membranes to maintain relatively stable intracellular environments Evidence Outcomes Students can: a. Analyze and interpret data to determine the energy requirements and/or rates of substance transport across cell membranes b. Compare organisms that live in freshwater and marine environments, and identify the challenges of osmotic regulation for these organisms c. Diagram the cell membrane schematically, and highlight receptor proteins as targets of hormones, neurotransmitters, or drugs that serve as active links between intra and extracellular environments d. Use tools to gather, view, analyze, and interpret data produced during scientific investigations that involve passive and active transport e. Use computer simulations and models to analyze cell transport mechanisms 21st Century Skills and Readiness Competencies Inquiry Questions: 1. What variables affect the rate of transport across a membrane? 2. Why is it important that cell membranes are selectively permeable? Relevance and Application: 1. Osmotically balanced solutions such as intravenous and ophthalmic solutions are critical in medical settings. 2. Drugs target receptor proteins such as hormones and neurotransmitters in membranes and mimic the action of natural signals there. 3. Technology is used to support humans on dialysis. Nature of Science: 1. Ask testable questions and make a falsifiable hypothesis about how cells transport materials into and out of the cell and use an inquiry approach to find the answer. 2. Share experimental data, and respectfully discuss conflicting results emulating the practice of scientists. 3. Recognize and describe the ethical traditions of science: value peer review; truthful reporting of methods and outcomes; making work public; and sharing a lens of professional skepticism when reviewing the work of others. Discussion question 2: How does the lesson you just participated in support professional expectations as seen in the new teacher evaluation tool? Work with 1 or 2 other people to highlight where you see this lesson aligning with the new teacher evaluation tool. • handout of part of the evaluation tool provided Record and discuss your findings. I. Professional Preparation Purposeful planning for instruction as evidenced by: • Knowledge of current District 11 curriculum and Unified School Improvement Plan (USIP) goals • Knowledge of subject • Knowledge of research-based best practices II. Professional Techniques and Strategies Demonstrates professional techniques that: • utilize effective teaching strategies that enhance student learning • establish a classroom environment conducive to learning Time to Reflect Please take a few minutes to reflect on your classroom experience today by responding (in writing – in your notebook) to the questions below: • How does the lesson you experienced today compare to lessons in your current classroom? (similarities? differences?) • What aspects of the lesson today would you like to incorporate into your classroom? The following series of slides contains additional information on osmosis / diffusion, including a few applications as well as related tutorials and youtube videos. Additional Info: Osmosis / Diffusion Osmosis The tendency for aqueous solutions [solvents] to migrate across a semi-permeable membrane from a higher water potential to a lower water potential is known as diffusion. Semi-permeable membranes are selective, allowing only specific particles to flow through and preventing others from invading. Water always flows from lower solute concentration [dilute solution] to higher solute concentration until a balance is produced. Dilute solutions are highly concentrated in water and low in solute. Hence, a low concentration of solute results in a high water potential. The general motion is from more water to less water or from less solute to more solute. Osmotic pressure During osmosis, pressure is generated due to the difference in solute potentials of two environments separated by a semi-permeable membrane. This pressure provides the force for the water to move from low solute concentration to high solute concentration. Once the water pressure reaches the osmotic pressure, osmosis stops. Additional Info: Osmosis / Diffusion The role of osmosis in living organisms Living cells may be thought of as microscopically small bags of solutions contained within semi-permeable membrane that allows water to flow in or out. In order for the cell to survive, the concentration of solutes within the cell cannot be changed dramatically. Water passes through the membrane in both directions to generate an equilibrium between the cell and its surroundings. If the cells are in a highly concentrated solution, the water in the cell would flow out to maintain the equilibrium between the exterior and interior of the cell. This may cause the cell to shrink due to loss of water and die of dehydration. Oppositely, if the cells are in a more diluted solution, water will enter the cell and cause it to burst and be destroyed. Additional Info: Osmosis / Diffusion Reverse Osmosis Reverse osmosis is the process by which excess pressure is placed on one end of a semi-permeable barrier in order to drive a solution from an area of high solute concentration to that of a low solute concentration. Opposite from general osmosis, the solvent does not go down a concentration gradient. In this case, the cell membrane serves as a filter. Reverse osmosis has been used industrially for water treatment. Salt water collected from the ocean is transformed to pure water by setting an external pressure equal to the air pressure at sea level. Water purification is only one industrial use of reverse osmosis. Metals and chemicals are also recycled through this process. Want to know more? http://www.youtube.com/watch?v=qucTUoHAeuY&feature=related Clark College Tutoring and Writing Center tutor Joey Smokey introduces the concept of diffusion and osmosis, providing multiple definitions and explaining how the two processes work. Along the way, he explains things such as concentration gradients, semipermeable membranes, colloids and suspensions, and the effects of concentration on red blood cells in isotonic, hypotonic, and hypertonic solutions. Related Youtube Videos http://www.youtube.com/watch?v=0c8acUE9Itw&feature=related An egg is placed in corn syrup for 60 minutes to show osmosis. The egg is then placed in fresh water to show the reverse effect. http://www.youtube.com/watch?v=o6nqYcrItiQ&feature=related Diffusion: Food Color and Water http://www.youtube.com/watch?v=2Th0PuORsWY&feature=related Diffusion through a membrane - Iodine turns blue in reaction to starch. When a starch solution is placed in dialysis tubing (a semipermeable membrane) and the tubing is added to a beaker of iodine , will the starch be able to pass through the tubing and turn the water blue or will the smaller iodine molecules pass through and turn the contents of the tube blue?