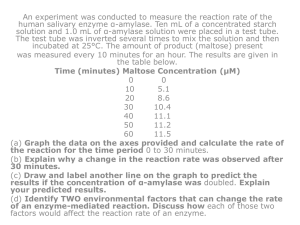
Enzymes & Photosynthesis
advertisement

Bioenergetics Graphing Tuesday • Create a line graph with 2 y axes. • These are fake numbers @ hunting in Summer Shade! Year # Hunters Year # Deer 2000 150 2000 8,000 2001 200 2001 7,800 2002 125 2002 3,000 2003 100 2003 2,500 2004 300 2004 3,000 2005 350 2005 3,250 2006 355 2006 4,500 Stem Cell Review • 1. What is a stem cell? _____________ ________ • 2. List the 2 types of stem cell: ______ ________ • 3. Which stem cell is controversial? Why? • 4. Where do they get adult stem cells from? Review • • • • Potential vs. Kinetic Energy List 4 macromolecule types How are these made/destroyed? Functions of Each Macromolecule. Metabolism • The sum of all chemical reactions occurring in an organism. • Catabolism- breaking down. EXERGONIC. Releases stored potential energy/heat. • Anabolism- building up. ENDERGONIC. Absorbs energy/heat from environment. • Anabolism and Catabolism are an example of ENERGY COUPLING…2 different processes united by common energy. Energy (E) • Kinetic- energy of movement, usually e- or protons in Biology. • Potential- energy of position, usually in the chemical bonds of e-/p in Biology. • Cell Respiration releases energy (KE), Photosynthesis allows capture of E from great E source (PE) Potential Energy vs. Kinetic Energy Thermodynamics • Study of heat and its properties. • First Law of Thermodynamics: energy cannot be created/destroyed just transformed/transferred. • Second Law of Thermodynamics: every energy transfer increases entropy (disorder). • Most organized at conception, as you move towards death you become more organized…evolution? Thermodynamics LE 8-3 Sunlight is high quality E, Heat is low quality E Heat Chemical energy First law of thermodynamics CO2 H2O Second law of thermodynamics Gibbs “Free” Energy- ability to work (make ATP/GTP) • Δ G = ΔH – TΔ S • G- Gibbs “free” energy • H – Enthalpy (Total usable energy in the system) • T – Temperature in Kelvin (273 + C⁰) • S- Entropy (Disorder created by something being broken down) • Δ – Change in a variable over time Unstable (Capable of work)=LIVING vs. Stable (no work)=DEAD G < 0 A closed hydroelectric system G = 0 LE 8-6a Catabolism if G is negative, e.g. cell respiration. There is free energy to do work Free energy Reactants Amount of energy released (G < 0) Final-initial E Energy Products Progress of the reaction Exergonic reaction: energy released LE 8-6b Anabolism if G is positive, then it cannot do work, energy is bound up (photosynthesis=endergonic) Free energy Products Energy Reactants Progress of the reaction Endergonic reaction: energy required Amount of energy required (G > 0) Remember • Not all energy can be used… • Lots is lost to heat, some to waste (defacation) Types of work performed by living cells Pi P Motor protein Protein moved Mechanical work: ATP phosphorylates motor proteins Membrane protein ADP + Pi ATP Pi P Solute transported Solute Transport work: ATP phosphorylates transport proteins P NH2 Glu + NH3 + Pi Glu Reactants: Glutamic acid and ammonia Product (glutamine) made Chemical work: ATP phosphorylates key reactants ATP ATP • The 3 PO4 make it very unstable. This instability allows it to do lots of work. Phosphorylation ATPADP +Pi ADP +Pi ATP G=-13J G=13J Exergonic, can do work Endergonic, can’t do work Phosphorus Cycle Initially in rocks, rocks weather, P then in soil or inwater to be used by producers to make phospholipids, DNA/RNA, proteins. U2,D1 Data Set 1 Picture Enzyme Review • Protein function is caused by structure…sequence of _ _ and how they are _. • All major processes in cells involve proteins. • Suffix of most proteins:_ • Proteins are catalysts: speed up and control rate of reactions. Enzyme Review • Enzymes are not consumed in the reaction. Benefit? • Enzymes used to be described as “lock and key” now they are said to be “induced fit” or “fits like a glove” • H bonds responsible for induced fit Enzymes Lower EA • Energy of Activation is the energy required to get the molecules lined up and ready for a reaction to take place (metabolism). • Because the molecules are sitting in the enzyme in position, it reduces all the time and energy of them “naturally” coming together. • Enzymes also eliminate the need for heat to move the molecules faster…we won’t incinerate ourselves during metabolism . Free energy Course of reaction without enzyme EA without enzyme EA with enzyme is lower Reactants Course of reaction with enzyme G is unaffected by enzyme Products Progress of the reaction . Substrate Active site Enzyme Enzyme-substrate complex Enzymatic Process • Active Site- location of chemical reactions between enzyme and substrate. • Enzyme Substrate Complex- caused by induced fit. Held together by H bonds, ionic bonds, and Van der Waals. • The amino acid R groups perform the reaction. R groups of Amino Acids . Substrates enter active site; enzyme changes shape so its active site embraces the substrates (induced fit). Substrates held in active site by weak interactions, such as hydrogen bonds and ionic bonds. Substrates Enzyme-substrate complex Active site is available for two new substrate molecules. Enzyme Products are released. Substrates are converted into products. Products Active site (and R groups of its amino acids) can lower EA and speed up a reaction by • acting as a template for substrate orientation, • stressing the substrates and stabilizing the transition state, • providing a favorable microenvironment, • participating directly in the catalytic reaction. 3 Factors that Affect Enzymes • • • • 1. Temperature 2. Salinity 3.pH *They all affect the 2*structure of proteins by altering the H bonds. • If a protein unwinds it is said to be __ • Type of protein that prevents misfolding_ Enzyme Inhibitors • These will slow or stop the rate of reactions • 1. Competitive Inhibitors- compete with substrate for active site, bind to active site, and SLOW reactions down. • 2. Non-competitive Inhibitors- bind somewhere to the enzyme, change the active site completely, and STOP reactions. • Inhibitors can be classified as reversible (Antabuse) or irreversible (Sarin-nerve gas) A substrate can bind normally to the active site of an enzyme. Substrate Active site . Enzyme Normal binding A competitive inhibitor mimics the substrate, competing for the active site. Competitive inhibitor Competitive inhibition A noncompetitive inhibitor binds to the enzyme away from the active site, altering the conformation of the enzyme so that its active site no longer functions. Noncompetitive inhibitor Noncompetitive inhibition Allosteric Enzymes “Allo” different, “stery” shape • Enzymes that will change shape, thus being turned off or on. • Inhibitor molecules turn the enzyme off • Feedback Inhibition or Negative Feedback Loop-prevents wasting energy • Activator molecules turn the enzyme on Feedback Inhibition or Negative Feedback Initial substrate (threonine) Active site available Threonine in active site Enzyme 1 (threonine deaminase) Isoleucine used up by cell Intermediate A Feedback inhibition Enzyme 2 Active site of enzyme 1 can’t bind Intermediate B theonine pathway off Enzyme 3 Isoleucine binds to allosteric site Intermediate C Enzyme 4 Intermediate D Enzyme 5 End product (isoleucine) Cooperativity • One active site helps other active sites on the same molecule. • RBC-4 part molecule, each part carries O. When Part 1 fills with O the next part does …and RBC deliveer O in the same way. • This is an example of cell efficiency/specializatino, conservation of E, and regulation. Proteins involved in constructing a red blood cell Quaternary Structure Polypeptide chain b Chains Iron Heme Polypeptide chain Collagen a Chains Hemoglobin Bioenergetics • Enzymes are needed in all efficient energy reactions. • Two energy reactions we will focus on: • Photosynthesis- anabolic, endergonic, +G • Cell Respiration-catabolic, exergonic, -G Remember • Electrons are a source of E • CHOs come from H20 and CO2 by plant’s chloroplast • E in a molecule is directly related to # H present. • Autotrophs = • Heterotrophs = Autotroph - Plants Autotroph - Algae Autotroph - Phytoplankton Autotroph - Bacteria Heterotroph - Animal Heterotroph - Fungus Photosynthesis • Chlorophyll- light absorbing protein pigment that reflects green light. Found in plants, algae, and blue-green bacteria. • Chloroplast- organelle that contains grana (thylakoids) and stroma Chloroplast Chloroplast Parts • Thylakoids- contain chlorophyll. Site of Light reaction. Purpose is to make ATP & NADPH. • Grana- stacks of thylakoids • Stroma- watery area @ thylakoids. Site of light independent (Calvin Cycle). Purpose is to use ATP & NADPH to make glucose using CO2 Photosynthesis chemical reaction (Remember… conservation of matter.) • 6 CO2 + 6 H2O C6H12O6 + 6 O2 + Heat Photosynthesis • Take radiant energy and convert into chemical energy (ATP & NADPH) • Take chemical energy (ATP & NADPH) and turn it into potential chemical energy (carbohydrate). Sugar creation is done by catabolism. Photosynthesis Light Reaction Photosynthesis Calvin Cycle Sunlight Terminology Electromagnetic Spectrum Absorption vs. Reflection Sunlight • • • • High quality E Sunlight travels in waves. Each color has a wavelength Red light has the longest wavelengths • Least energy of the white light • Blue light has the shortest wavelengths • Most energy of the white • Units of light are called photons Chloroplasts REFLECTING Green Light White light Refracting prism Chlorophyll solution Photoelectric tube Galvanometer 0 Slit moves to pass light of selected wavelength Green light 100 The high transmittance (low absorption) reading indicates that chlorophyll absorbs very little green light. Chlorophyll ABSORBING Blue light to power photosynthesis White light Refracting prism Chlorophyll solution Photoelectric tube 0 Slit moves to pass light of selected wavelength Blue light 100 The low transmittance (high absorption) reading indicates that chlorophyll absorbs most blue light. Chloroplasts absorbing the blue and the red light waves. The green is NOT being absorbed. Light Absorption vs. Reflection • Absorbed light = used light (red and blue0 • Reflected light- unused light (green light) in plants Chlorophyll Molecule (How many electrons are in Mg’s outer shell?) Hint: Look at the Periodic Table. Absorbed Light • Light is absorbed by pigments: • Chlorophyll A-main one • Chlorophyll B- help A • Carotenoids- reflects orange, red, yellow, help A • Photosystems- groups of pigments in the thylakoid membrane • Photosystem I: makes ATP & NADPH • Photosystem II: makes ATP Photosystem and collecting sunlight energy. Where are the photosystems located? Synthesis Question (U2, D6) • Question: The word “photosynthesis” means the “the process of using light to make”. What is made in the process is the organic macromolecule sugar (carbohydrate). In no more than three sentences, justify the meaning of photosynthesis by briefly telling what colors of light are involved in the process, what the light is converted into, and what are those molecules purpose. (5 Points) • • • • • 1pt. Discussion of the red and blue colors of white light being absorbed by plants. 1pt. Discussion of converting the light energy into ATP and NADPH or chemical energy molecules 1pt. Discussion of ATP and NADPH (Chemical energy molecules) being used to make sugar. 1pt. Correct use of scientific terms. 1pt. Answer has no more than three sentences. (Following Directions.) Remember • Cells have a high SA:V ratio. Why? SA:V ratio also high for mitochondria and chloroplast. • Valence electrons involved in bonding. Light Dependent Reactions of Photosynthesis • * Turns radiant energy into chemical energy __ & __. • Takes place in the light, on thylakoid membrane. • Uses photosystems either in a cyclic electron flow or a non-cyclic electron flow. • There are 1000s of photosystems per each thylakoid. Benefit? SA:V? Non-cyclic electron flow Cyclic electron flow Photosynthesis • 1. Sunlight strikes the Photosystem II, 2 H2O enters Photosystem II. • 2. O2 is released from PII as waste, and 2H+, 2 E- are left. • 3. H+ is in the stroma, and the e- move using a carrier protein, Cytochrome C, down the primary electron transport chain. • 4. Light also strikes Photosystem I causing it to lose electrons and move down another primary electron transport chain. • 5. e-from PI, move towards enzyme, to NADP+ Reductase this enzyme reduces NADP+ into NADPH. • Redox Reactions- 2 molecules exchanging e- • 6. Redox reactions cause e- to move down ETC • 7. As e- move down the ETC, they power proton pumps (H+) with their kinetic energy. • 8. H+ actively pumped from stroma into the thylakoid which causes a change in pH, and the concentration gradient is established. (air in balloon) • 9. This [gradient] is the potential energy that will make ATP using the enzyme ATP Synthetase Complex (complex=many proteins) through anabolic phosphorylation. (air leaving balloon) • The quantities are mind boggling. A hectare (e.g. a field 100 m by 100 m) of wheat can convert as much as 10,000 kg of carbon from carbon dioxide into the carbon of sugar in a year, giving a total yield of 25,000 kg of sugar per year. • There is a total of 7000 x 109 tonnes of carbon dioxide in the atmosphere and photosynthesis fixes 100 x 109 tonnes per year. So 15% of the total carbon dioxide in the atmosphere moves into photosynthetic organisms each year. H+ (protons) being pumped into the thylakoid to “build” potential energy. Photosynthesis Energy Coupling • Using energy from the proton pump to make energy in the form of ATP. • Active transport sets up [gradient], diffusion creates the ATP • Making ATP in photosynthesis is called chemiosmosis. Data set 2 picture (U2,D7) Review Remember • 1. Law of Conservation of Mass- Matter is neither created or destroyed…just transferred/ transformed. • CHO are energy storage molecules for quick release. • C is the backbone of the 4 biomolecules. • Primary source of C is CO2 from air. Light Independent ReactionsCalvin Cycle • Uses ATP and NADPH to perform carbon fixation (make sugar from CO2). • 1. CO2 enters through the stomata, CO2 diffuses through c.m. and membrane of chloroplast into the stroma. • 2. 3CO2 molecules will be added to RuBPa five carbon molecule. • 3. Immediately the 6C molecule breaks into 2 3C molecules (6 3C molecules total). Calvin Cycle step 1 • 4. Use 6 ATP & 6 NADPH to bend each 3C sugars. (6 3C sugars). • The bent 3C sugars are then 6 molecules of G3P. • 5. 1G3P goes into making glucose, the other 5 G3Ps go back into the Calvin Cycle. • 6. Using 3 ATP they are converted into 3 molecules of RuBP Making Glucose • One G3P per turn of the cycle. • Takes 2 turns to make one glucose. • Takes 9 ATP and 6NADPH per turn… 18 ATP and 12 NADPH per glucose. • The glucose is used for food, and excessis stored in starch to be used in cell respiration or making cell walls. Photorespiration • Uses O2 to fix carbon instead of CO2. • This is a last resort to stay alive, when the stomata are closed off to prevent H20 loss. • In C3 plants this will quickly lead to death. • In C4 plants there is extra enzymes to grab CO2, and photosynthesis occurs in the inner leaf cells. These plants are adapted for hot weather…corn, cotton, summer flowers. CAM Plants • Crussulacean Acid Metabolism- utilize CO2 stored as Crussulacean Acid because stomata only open at night. The C. acid is broken down in the day, and releases CO2 for Calvin Cycle. • Desert plants, succulents, bromeliads, etc. • CAM Plants prevent transpiration. Transpiration • Transpiration dictates available energy… • Deserts have lots of transpiration …minimal photosynthesis…minimal E. • Rainforests have little transpiration…lots of photosynthesis…lots of E…bigger food webs. Competition vs. Evolution • Each plant type (C3, C4, CAM) have its own niche. • A niche prevents competition thus conserving E. • The more E conserved the more spent of reproducing, thus highly populating the area. • Is this competition or evolution? Justify in 3 sentences. Remember… • Law of Conservation of Matter… • Second Law of Thermodynamics- all E initiates from the sun (high quality), and ends up in entropy (low quality/disorder). • Carbon skeletons for 4 biomolecules Energy Flow and Matter Cycling Tertiary consumers Microorganisms and other detritivores Detritus Secondary consumers Primary consumers Primary producers Heat Key Chemical cycling Energy flow Sun Carbon Cycle 1. All C starts in atm. 2. Photosynthesis fixes CO2 to sugar. 3. Sugars used by consumers in cell respiration and release CO2. 4. Fossil fuel burning also releases CO2 into atm. CO2 in atmosphere Photosynthesis Cellular respiration Burning of fossil fuels and wood Carbon compounds in water Higher-level Primary consumers consumers Detritus Decomposition Ecosystems • All the interacting communities is a given area, also involves abiotic factors. • Important Abiotic factors: • • • • Temp. Water Nutrient cycling Energy flow Trophic Structure “troph=feed” • These are feeding relationships. • Second Law- with each level E is lost to entropy. • All E eventually lost to heat. • Matter also flows through the trophic levels, never created/ destroyed…think geochemical cycles Food Web vs. Chain Energetic Hypothesis/ Pyramid of Numbers • Energetics Hypothesis- there are short food chains because of the 10% rule. • 90% of all energy consumed by the organisms is lost to heat/ maintenance before eaten by the next trophic level. Food chains and the 10% Rule of Energy 10% Rule Plant material eaten by caterpillar 200 J 67 J Feces 100 J 33 J Growth (new biomass) Cellular respiration Primary Productivity • Total amount of sunlight turned into chemical energy by photosynthesis. • Global Energy Budget- amount of sunlight used for photosynthesis. • Photosynthesis produces 170 billion tons of sugar annually. • Using only 1% of solar energy. Productivity of the Earth (Based on Chlorophyll Density) Red And Yellow areas have the highest productivity…so where are they located? Net Primary Productivity • Gross Primary Productivity- total E produced • R- E used by autotrophs • NPP usually = 10%. It is the E available to next trophic level. • NPP = GPP - R Data Set 3 picture U2,D9