PowerPoint
advertisement

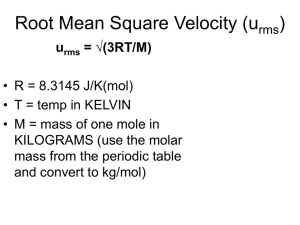
Module 5.04 Gas Stoichiometry Stoichiometry Using the Ideal Gas Law • If you are given the volume, pressure and temperature of a gaseous reaction you can use the ideal gas law to solve for moles before solving a stoichiometry problem. • n = PV RT n = # of moles P = Pressure in atm V = Volume in Liters R = Ideal gas constant in 0.0821 L x atm Mol x K Stoichiometry • It’s a process that allows us to mathematically convert and calculate the relationships between the amount of reactants and products in a chemical reaction. Example 1 (Ideal gas law before stoichiometry) • How many grams of sodium chloride can react with 18.3 liters of fluorine gas at 1.2 atmospheres at 299 kelvins? F2(g) + 2 NaCL(s) Cl2 (g) + 2NaF (s) 1. Make sure your equation is balance 2. Always write down what you know n=PxV RxT V = 18.3 L P = 1.2 atm T = 299 K R = 0.0821 L atm Mol K Example 1 (con’t) n=PxV RxT n(F2) = 1.2 atm x 18.3 L 0.0821 x 299K n(F2)= 0.895 mol F2 Now that you have the amount of F2 in moles, you can start the stoichiometry problem. 0.895 mol F2 x 2 mol NaCl x 58.44 g NaCl = 105 g NaCl 1 mol F2 1 mol NaCl Practice 1 How many grams of sodium metal are needed to react completely with 17.8 liters of chlorine gas at 297 kelvins and 1.25 atmospheres? 2Na(s) + Cl2(g) 2 NaCl(s) Example 2 (Ideal gas law after stoichiometry) If you are asked to solve for the volume or pressure of a gaseous Product, you can first solve for moles of that product using stoichiometry and then plug that value into the ideal gas law. V = nRT or P P = nRT V Example 2 If 52.0 g of magnesium metal react with excess hydrochloric acid, how many liters of hydrogen gas can be produced at 27 C And 0.97 atmospheres? Mg(s) + 2HCl(aq) H2(g) + MgCl2 (aq) 1 mol Mg x 1 mol H2 52.0 g Mg x 24.3 g Mg 1 mol Mg V= (2.14 mol H2)(0.0821)(300 K) 0.97 atm V (H2) = 54.3 L of H2 gas = 2.14 mol H2 Practice 2 If 45.8 grams of lithium metal react with excess water, how many liters of hydrogen gas will be produced at 27.5 C and 1.35 atmospheres? 2 Li (s) + 2 H2O (l) 2 NaOH (aq) + H2 (g) Mole Ratios and Coefficients 2 CO (g) + O2 (g) 2 CO2 (g) The coefficients in this balanced equation provide a volume ration between gases when they are at the same temperature and pressure. A given volume of oxygen gas will react with twice that volume of carbon dioxide, because the ration of O2 to CO is 1:2 (given by the coefficients) The coefficients provide volume ratios (only gaseous reactants and products) that can be used in stoichiometry calculations involving volume. Practice 1 2 CO (g) + O2 (g) 2 CO2 (g) Assuming all volume measurements are made at the same temperature and pressure, how many milliliters of carbon dioxide gas be produced when 206.5 milliliters of oxygen gas react with excess carbon monoxide? Practice 2 C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) How many liters of propane gas (C3H8) need to be combusted to produce 3.8 liters of carbon dioxide, if all measurements are taken at the same temperature and pressure? Temperature and Pressure STP = standard temperature and pressure T = 273.15 kelvins P = 1.0 atm 1 mol of gas 22.4 L or 22.4 L 1 mol of gas These ratios can be used within a stoichiometry calculation whenever conditions are at STP This relationship between volume and moles replaces the need to use the ideal gas law. Practice 1 How many grams of calcium oxide (lime) would be produced when 50.0 liters of carbon dioxide is produced at STP? CaCO3 (s) CaO (s) + CO2 (g) Diffusion and Effusion Diffusion - The gradual mixing of two gases because of the spontaneous, random motion of their particles. Effusion - The movement of gas particles through a small opening in the container wall due to the pressure and particle movement inside the container. The relationship between mass and velocity is expressed by the gas law known as Graham’s Law: √ Molar mass of B Rate of effusion of A = Rate of effusion of B √ Molar mass of A Variations of Graham’s Law rate of effusion of A rate of effusion of B = √ Molar mass of B √ Molar mass of A √ Molar mass of B velocity of A = velocity of B √ Molar mass of A rate of diffusion of A rate of diffusion of B = √ Molar mass of B √ Molar mass of A Practice 1 (effusion) A sample of hydrogen gas (H2) effuses through a porous 7.96 times faster than an unknown gas. What is the molar mass of the unknown gas? Practice 2 (diffusion) Under certain conditions argon (Ar) gas diffuses at a rate of 5.2 centimeters per second. Under the same conditions, an unknown gas diffuses at a rate of 3.5 centimeters per second. What is the approximate molar mass of the unknown gas? Practice 3 Which sample of gas would effuse the fastest under the same temperature and pressure conditions? Use the periodic table to help answer this question. CH4 Cl2 O2 Ne