Ch5 Modern Periodic Table - Saint Joseph Regional High School
advertisement

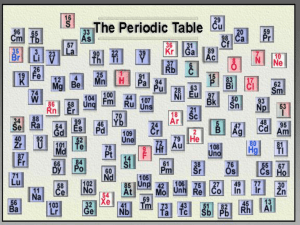
5.2 The Modern Periodic Table The eight-note interval between any two notes on a keyboard with the same name is an octave. The sounds of musical notes that are separated by an octave are related, but they are not identical. In a similar way, elements in the same column of the modern periodic table are related but not identical. 5.2 The Modern Periodic Table The Periodic Law How is the modern periodic table organized? 5.2 The Modern Periodic Table The Periodic Law How is the modern periodic table organized? In the modern periodic table, elements are arranged by increasing atomic number (number of protons). 5.2 The Modern Periodic Table The Periodic Law How is the modern periodic table organized? In the modern periodic table, elements are arranged by increasing atomic number (number of protons). Properties of elements repeat in a predictable way when atomic numbers are used to arrange elements into groups. 5.2 The Modern Periodic Table The Periodic Law The modern periodic table is based on atomic number, or number of protons. 5.2 The Modern Periodic Table The Periodic Law Periods Each row in the table of elements is a period. • Hydrogen, the first element in Period 1, has one electron in its first energy level. • Lithium, the first element in Period 2, has one electron in its second energy level. • Sodium, the first element in Period 3, has one electron in its third energy level. • This pattern applies to all the elements in the first column on the table. 5.2 The Modern Periodic Table The Periodic Law Groups Each column in the periodic table is called a group. • The elements in a group have similar electron configurations, so members of a group in the periodic table have similar chemical properties. • This pattern of repeating properties is the periodic law. 5.2 The Modern Periodic Table The Periodic Law Periodic Table of the Elements 5.2 The Modern Periodic Table Atomic Mass What does the atomic mass of an element depend on? Atomic mass is a value that depends on the distribution of an element’s isotopes in nature and the masses of those isotopes. 5.2 The Modern Periodic Table Atomic Mass Atomic Mass Units The mass of an atom in grams is extremely small. In order to have a convenient way to compare the masses of atoms, scientists chose one isotope to serve as a standard. • Scientists assigned 12 atomic mass units to the carbon-12 atom, which has 6 protons and 6 neutrons. • An atomic mass unit (amu) is defined as one twelfth the mass of a carbon-12 atom. 5.2 The Modern Periodic Table Atomic Mass There are four pieces of information for each element. 5.2 The Modern Periodic Table Atomic Mass There are four pieces of information for each element. Atomic number 5.2 The Modern Periodic Table Atomic Mass There are four pieces of information for each element. Atomic number Element symbol 5.2 The Modern Periodic Table Atomic Mass There are four pieces of information for each element. Atomic number Element symbol Element name 5.2 The Modern Periodic Table Atomic Mass There are four pieces of information for each element. Atomic number Element symbol Element name Atomic mass 5.2 The Modern Periodic Table Atomic Mass Isotopes of Chlorine In nature, most elements exist as a mixture of two or more isotopes. The element chlorine has an atomic mass of 35.453 amu. Where does the number 35.453 come from? • There are two natural isotopes of chlorine, chlorine-35 and chlorine-37. • An atom of chlorine-35 has 17 protons and 18 neutrons. • An atom of chlorine-37 has 17 protons and 20 neutrons. 5.2 The Modern Periodic Table Atomic Mass Weighted Averages This table shows the atomic masses for the two naturally occurring chlorine isotopes. The value of the atomic mass for chlorine is a weighted average. If you add the atomic masses of the isotopes and divide by 2, you get 35.967, not 35.453. 5.2 The Modern Periodic Table Classes of Elements What categories are used to classify elements on the periodic table? 5.2 The Modern Periodic Table Classes of Elements Elements are classified as metals, nonmetals, and metalloids. 5.2 The Modern Periodic Table Classes of Elements The periodic table presents three different ways to classify elements. • State: solid—black symbol, liquid—purple symbol, or gas—red symbol • Occurrence in nature: elements that do not occur naturally—white symbol. • General properties: metal—blue background, nonmetal—yellow background, or metalloid—green background 5.2 The Modern Periodic Table Classes of Elements Metals The majority of the elements on the periodic table are classified as metals. Metals are elements that are good conductors of electric current and heat. • Except for mercury, metals are solids at room temperature. • Most metals are malleable. • Many metals are ductile; that is, they can be drawn into thin wires. 5.2 The Modern Periodic Table Classes of Elements A When magnesium reacts with oxygen, a dull layer forms on its surface. The layer can be removed to reveal magnesium’s shiny surface. B Many telescope mirrors are coated with aluminum to produce a surface that reflects light extremely well. 5.2 The Modern Periodic Table Classes of Elements The metals in groups 3 through 12 are called transition metals. Transition metals are elements that form a bridge between the elements on the left and right sides of the table. • Transition elements, such as copper and silver, were among the first elements discovered. • One property of many transition metals is their ability to form compounds with distinctive colors. 5.2 The Modern Periodic Table Classes of Elements A compound of oxygen and the transition element erbium is used to tint the pink glass lenses. 5.2 The Modern Periodic Table Classes of Elements Nonmetals Nonmetals generally have properties opposite to those of metals. • Nonmetals are elements that are poor conductors of heat and electric current. • Nonmetals have low boiling points–many nonmetals are gases at room temperature. • Nonmetals that are solids at room temperature tend to be brittle. If they are hit with a hammer, they shatter or crumble. 5.2 The Modern Periodic Table Classes of Elements Fluorine is the most reactive nonmetal. The gases in Group 18 are the least reactive elements in the table. Some toothpastes use a compound of the nonmetal fluorine and the metal sodium to help prevent tooth decay. 5.2 The Modern Periodic Table Classes of Elements Metalloids Metalloid elements are located on the periodic table between metals and nonmetals. • Metalloids are elements with properties that fall between those of metals and nonmetals. • For example, a metalloid’s ability to conduct electric current varies with temperature. Silicon (Si) and germanium (Ge) are good insulators at low temperatures and good conductors at high temperatures. 5.2 The Modern Periodic Table Variations Across a Period How do properties vary across a period in the periodic table? 5.2 The Modern Periodic Table Variations Across a Period Across a period from left to right, the elements become less metallic and more nonmetallic in their properties. 5.2 The Modern Periodic Table Variations Across a Period From left to right across Period 3, there are three metals (Na, Mg, and Al), one metalloid (Si), and four nonmetals (P, S, Cl, and Ar). 5.2 The Modern Periodic Table Variations Across a Period • Sodium reacts violently with water. • Magnesium will not react with water unless the water is hot. • Aluminum does not react with water, but it does react with oxygen. • Silicon is generally unreactive. • Phosphorus and sulfur do not react with water, but they do react with oxygen. • Chlorine is highly reactive. • Argon hardly reacts at all. 5.2 The Modern Periodic Table Assessment Questions 1. What determines the atomic mass of an element? a. the natural distribution of isotopes and the atomic numbers of those isotopes b. the natural distribution of isotopes and the masses of those isotopes c. the mass of the isotope of the element that has the most neutrons d. the average number of protons in the element’s nucleus 5.2 The Modern Periodic Table Assessment Questions 1. What determines the atomic mass of an element? a. the natural distribution of isotopes and the atomic numbers of those isotopes b. the natural distribution of isotopes and the masses of those isotopes c. the mass of the isotope of the element that has the most neutrons d. the average number of protons in the element’s nucleus ANS: B 5.2 The Modern Periodic Table Assessment Questions 2. Which of the following is not characteristic of metals? a. b. c. d. ductile good electrical conductor typically solid at room temperature brittle 5.2 The Modern Periodic Table Assessment Questions 2. Which of the following is not characteristic of metals? a. b. c. d. ductile good electrical conductor typically solid at room temperature brittle ANS: D 5.2 The Modern Periodic Table Assessment Questions 3. Within a period of the periodic table, how do the properties of the elements vary? a. b. c. d. Metallic characteristics increase from left to right. Metallic characteristics decrease from left to right. Reactivity increases from left to right. Reactivity decreases from left to right. 5.2 The Modern Periodic Table Assessment Questions 3. Within a period of the periodic table, how do the properties of the elements vary? a. b. c. d. Metallic characteristics increase from left to right. Metallic characteristics decrease from left to right. Reactivity increases from left to right. Reactivity decreases from left to right. ANS: B 5.2 The Modern Periodic Table Assessment Questions 1. In the modern periodic table, elements are arranged in order of increasing atomic mass. True False 5.2 The Modern Periodic Table Assessment Questions 1. In the modern periodic table, elements are arranged in order of increasing atomic mass. True False ANS: F, atomic number