Periodic Table
advertisement

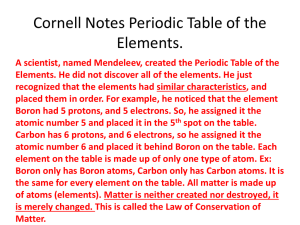
1790’s French chemist Antoine-Laurent Lavoisier made the first periodic table. 1803- John Dalton improved the periodic table by assigning symbols and masses to each element. 1869- Dimitri Mendeleev, a Russian chemist, arranged the 63 known element at the time, into groups based on their chemical properties and atomic weights. 1912- Henry Moseley, an English physicist improved Mendeleev’s table by listing the elements by their atomic number. 1940’s- Glenn Seaborg discovered plutonium and all the transuaranic elements from 94 to 102. He was awarded the Nobel Prize in chemistry for his work in 1951. Element 106 has been named seaborgium (Sg) in his honor. Today’s periodic table: arranged by atomic number groups-columns of elements having similar chemical properties periods-in order of atomic number, increase across the period or row Periodic Table Periodic Law When elements on the periodic table are arranged by atomic number, relationships and similarities in properties can be seen. This means that when the elements from the periodic table are arranged in a certain way from lowest to highest atomic number (the number of protons), the ones near each other will have similar properties! The_Periodic_Table.asf What do elements in the same group have in common? They have the same number of valence electrons and other similar chemical and physical properties. They all have the same number of electrons in the outer shell and thus react similarly. For example, all the group 1 (Alkali metals) elements react violently with water. From left to right in a period, explain how the atoms change. Each row or period of the periodic table corresponds to the number of electron shells in an atom of the elements in that row. The elements in the second period each have two electron shells, and the elements in the sixth period have six electron shells. Rough draft reports due tomorrow............... The Periodic Table I. History A. Lavoisier, Dalton B. Mendeleev, Meyer C. Rutherford, Moseley D. Seaborg II Organization A. Periods B. Groups C. Group names and info III. Metals, Nonmetals, Metalloids Text pages 98 - 103 Read handout on line..... IV. New Elements A. Identifying B. Naming the new elements CALCULATORS NEEDED IN CLASS FOR WEDNESDAY! BE SURE AND BRING YOUR OWN! Elements fall into three groups characterized by similar properties. metals, nonmetals, and metalloids METALS: majority of elements…where on the periodic table??? Shiny luster Good conductors of heat and electricity Solid at room temperature Malleable, or can be shaped Ductile, or can be drawn into wires without breaking 3/4 of all elements are metals Most metals have one, two or three electrons in their outer shell During chemical reactions, metal atoms lose their outer electrons to other atoms, usually, non metals. The outer electrons are far from the positive pull of the protons, thus held loosely in place. A metal atom becomes stable, or nonreactive, when it has lost its outer electron. NONMETALS how many elements and where on the periodic table??? Dull in appearance Poor conductors of heat and electricity Many are gases at room temperature Brittle, cannot change shape without breaking 96% of the human body is made up of nonmetals METALLOIDS: how many elements and where on the periodic table??? Have characteristics of both metals and nonmetals Do not conduct heat and electricity as well as metals All are solids at room temperature Group Information on the Periodic Table The periodic table groups are as follows (in the brackets are shown the old systems: European and American): Group 1 (IA,IA): the Alkali metals Group 2 (IIA,IIA): the Alkaline earth metals Group 3 (IIIA,IIIB) Group 4 (IVA,IVAB) Group 5 Group 6 Group 7 Group 8 Group 9 Group 10 Group 11 Group 12 (IIB,IIB) Group 13 (IIIB,IIIA): the Boron Group Group 14 (IVB,IVA): the Carbon Group Group 15 (VB,VA): Nitrogen Group Group 16 (VIB,VIA): the Chalcogens Group 17 (VIIB,VIIA): the Halogens Group 18 (Group 0): the Noble gases Group 1: The alkali metals are lithium, sodium, potassium, rubidium, cesium, and francium. Hydrogen is also part of this group, but is only in a metal state at great depths within Jupiter, the pressure is so great that the hydrogen atoms are broken up and the electrons are freed so that the resulting atoms consist of bare protons. This produces a state in which the hydrogen becomes metallic. Alkali Metal Properties Lower densities than other metals One loosely bound valence electron Largest atomic radii in their periods Low ionization energies Low electronegativities Group 2: The alkaline earth metals All the elements are all metals with a shiny, silvery- white color. They are all soft, low density metals, which react readily with halgens to form ionic salts, and with water but not as rapidly as the alkaline metals. For example, sodium and potassium react with room temperature water, however, magnesium reacts only with steam and calcium reacts only with hot water. The elements have two electrons in their valance, or outermost shell. 38 elements in groups 3 through 12 of the periodic table are called "transition metals". As with all metals, the transition elements are both ductile and malleable, and conduct electricity and heat. The interesting thing about transition metals is that their valence electrons, or the electrons they use to combine with other elements, are present in more than one shell. This is the reason why they often exhibit several common oxidation states. There are three noteworthy elements in the transition metals family. These elements are iron, cobalt, and nickel, and they are the only elements known to produce a magnetic field. The Boron Group, Group 13 Except for boron itself, which is a nonmetal, the elements of group IIIA, or Group 13, are metals (aluminum, gallium, indium, thallium). Even boron in its elemental state is a hard gray material that might be mistaken for a metal. The elements of group IIIA have a valence (number of bonds to atoms other elements) of three. There are exceptions. Thallium often has a valence of 1 as well as of 3. Boron often has a valence of 4 (bonds) because of filling the empty space in its octet from other species from outside. As a group, the elements of the boron group decrease in electronegativity as one goes down the column. Group 14: The Carbon Group has the elements change from nonmetallic in character at the top of the Group to metallic at the bottom. Carbon is a non-metal, silicon and germanium are metalloids, and tin and lead are typical metals. The general reactivity of the Group as a whole is therefore difficult to ascertain, and the reactivity of each element must be considered individually. Diamond has a very high refractive index (the reason for its sparkle) and this, along with its rarity, has made it valuable as a jewel. However, it is also the hardest natural substance known and so is important industrially. The most important physical property of silicon is that it is a semiconductor. Small silicon chips, just a few millimetres square, have revolutionised the computer and microprocessor industries. Tin and lead, as typical metals, are good conductors of electricity. Group 15: Nitrogen Group includes nitrogen, phosphorus, arsenic, antimony and bismuth and ununpentium. The name pnictogens is also sometimes used for this group; it is not approved by IUPAC . The spelling pnicogen is also recorded. Both spellings derive from the Greek πνίγειν (pnigein), to choke or stifle, which is a property of nitrogen. The chalcogens are the name for the periodic table group 16. It consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), the radioactive polonium (Po), and the synthetic ununhexium (Uuh). Oxygen and sulfur are nonmetals, polonium is a true metal, and selenium and tellurium are metalloid semiconductors Nevertheless, tellurium, as well as selenium, is often referred to as a metal when in elemental form. Chalcogenides are quite common as minerals. For example, FeS2 ( pyrite ). The mineral pyrite or iron pyrite is iron disulfide, FeS. It has isometric crystals that usually appear as cubes or pyritohedrons. It has a slightly uneven and conchoidal fracture, a hardness of 6-6. 5, and a specific gravity of 4. Its metallic luster and) is an iron ore and AuTe2 gave its name to the gold rush town of Telluride, Colorado Group 17: the halogens are a chemical series including: fluorine , chlorine, bromine, iodine, and astatine. The word comes from Greek roots meaning "salt" and "creator". These elements are diatomic molecules in their natural form. They require one more electron to fill their outer electron shells , and so have a tendency to form a singlycharged negative ion. This negative ion is referred to as a halide ion; salts For other meanings of the word salt see salt (disambiguation In chemistry, a salt is a composed of positively charged cations and negatively charged anions, so that the product is neutral and without a net charge. They are typically the product of a chemical containing these ions are known as halides. A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen. Most salts are halides. Group 18: the noble gases are a chemical series which include the elements helium , neon , argon , krypton , xenon and radon . The term noble gas comes from the fact that, just like the common view of human nobility, these gases generally sit around not doing anything, and avoid reacting with 'common' elements. The noble gases were previously referred to as inert gases, but this term is not strictly accurate now that some have been shown to take part in chemical reactions. Because of their unreactivity, the noble gases were not discovered until the existence of helium was hypothetically deduced from a spectrographic analysis of the sun, and later on proven when William Ramsay isolated it. The noble gases also have very weak inter-atomic forces of attraction, and consequently very low melting point. For the Periodic Table: 1. A copy of the Periodic Table with a key to describe what information is given. 2. Color code the Table as to solids, liquids, gases 3. Label periods and groups 4. Include a report on how the Periodic table came to be....include all the people who were responsible for it’s creation. Use your textbook, encyclopedia, or other sources. FINAL draft reports due tomorrow............... The Periodic Table I. History A. Lavoisier, Dalton B. Mendeleev, Meyer C. Rutherford, Moseley D. Seaborg II Organization A. Periods B. Groups C. Group names and info III. Metals, Nonmetals, Metalloids IV. New Elements A. Identifying B. Naming the new elements Have you ever wondered what rhyme or reason there is for the placement of elements in the Periodic Table of the Elements? Believe it or not, this strange arrangement of elements and data makes perfect sense (if we know what to look for). In this activity, we will again practice identifying the numbers of protons, neutrons, and electrons in the atoms of various elements. We will also make sketches of atoms using Neils Bohr’s atomic model. Finally, we will arrange these atom drawings into the first three periods (or rows) of the Periodic Table of the Elements. By the end of this long process, we should be able to safely state that the organization of the Periodic Table of the Elements does, indeed, make sense. Part A: Use the table below to help organize all of the key data for the first 18 elements of the Periodic Table of the Elements. Atomi Element Name c Numb er 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Elemen Number t of Symb Protons ol Number Numbe Mass of Number r of Electro Neutr ns ons Part B: Once Part A is finished, we will now make correct Bohr model drawings of atoms of each of these 18 elements on the sectioned off sheets of paper provided. Using compasses or other available circle drawing tools, make these sketches neatly and be sure to include the NAME and SYMBOL of each element in addition to the details in the model. See the example below. Part C - checklist of finishing touches: Cut out all 18 of the atom drawings and lay them out on the large sheet of paper in the order they are placed in the Periodic Table. PLEASE, DO NOT GLUE ANYTHING IN PLACE until checked. Have your seat mate check your project before you glue. Glue the atom drawings to your Periodic Table of the Elements. Color-code the electron energy levels. The first (innermost) level will be one color, the second level another color, and the third level a third color. Circle ONLY the OUTER electrons in each atom. Number GROUPS going across the top. Number PERIODS going down the side. Give it a neat and appropriate title. ___ Answer all ANALYSIS PROBLEMS on the sheet provided. Please respond to the following problems completely in the spaces provided below them. Explain what similarity each of the atoms in a particular group share. Look carefully at your Periodic Table of the Elements and look for a pattern in the structures of these elemental atoms. NOTE: Atoms prefer to be in a state that is stable; in other words ,with a full outer energy level. They seek to do so by either GAINING electrons to fill a nearly full outer energy level or by LOSING electrons to empty out a nearly empty energy level. 2a. Using the information above, explain why it is that sodium (Na) so easily bonds (or reacts) with chlorine (Cl). 2b. Do you think that lithium (Li) would want to bond with chlorine (Cl)? Explain. 2c. The elements in Group 18 are referred to as noble or inert gases. They are particularly special because they do not like to react with other elements. Explain why it is that these elements are so non-reactive. 3. Explain what similarity each of the atoms in a particular period share. Look carefully at your Periodic Table of the Elements and look for a pattern in the structures of these elemental atoms. 4. Using what has been learned in this assignment, answer these 3 questions about the element in Period 4, Group 2: a. How many outer electrons does each atom have? b. How many energy levels does it have? c. Name an element that would behave in a similar way. 5. In the space below, make a Bohr model drawing of the element with atomic number 19. Next to your drawing, be sure to include the atomic number, name, symbol, and mass numbe of the element. 6. Name one atom (element) that the atom you drew in problem 5 would likely react with. Explain why the atom you drew in problem 5 would choose this particular element with which to react. Use drawings if that would help your explanation. 7. THINK HARD! Magnesium chloride (MgCl2) is a compound that is formed by the reaction of one magnesium (Mg) atom with two chlorine (Cl) atoms. Using what you have learned in this project, explain why these atoms would want to combine to form one compound. Use drawings to help explain your thinking. 5. In the space below, make a Bohr model drawing of the element with atomic number 19. Next to your drawing, be sure to include the atomic number, name, symbol, and mass number of the element.number 19 Next to your drawing, be sure to include the atomic number, name, symbol, and mass number of the element. 6. Name one atom (element) that the atom you drew in problem 5 would likely react with. Explain why the atom you drew in problem 5 would choose this particular element with which to react. Use drawings if that would help your explanation. 7. THINK HARD! Magnesium chloride (MgCl2) is a compound that is formed by the reaction of one magnesium (Mg) atom with two chlorine (Cl) atoms. Using what you have learned in this project, explain why these atoms would want to combine to form one compound. Use drawings to help explain your thinking. Attachments The_Periodic_Table.asf