Lecture 10
advertisement

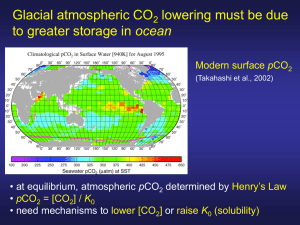
Lecture 10: Ocean Carbonate Chemistry: Ocean Distributions Ocean Distributions Controls on Distributions What is the distribution of CO2 added to the ocean? See Section 4.4 Emerson and Hedges Sarmiento and Gruber (2002) Sinks for Anthropogenic Carbon Physics Today August 2002 30-36 CO2 + rocks = HCO3- + clays CO2 Gas Exchange Atm River Flux Ocn CO2 → H2CO3 → HCO3- → CO32- Upwelling/ Mixing + H2O = CH2O + O2 + Ca2+ = CaCO3 CO2 BorgC BCaCO3 Biological Pump Controls: pH of ocean (controlled by DIC and Alk) Sediment diagenesis Influences on pCO2 Ko: Solubility of CO2 (same as KH) K1, K2: Dissociation constants Function of Temperature, Salinity Depends on biology and gas exchange Depends on biology only Derive starting with: CO2(g) + CO32- = 2 HCO3And use alk – DIC ~ CO32- and 2DIC – alk ~ HCO3- Ocean Distributions – versus depth, versus ocean Atlantic Pacific Points: See Key et al (2004) GBC 1. Uniform surface concentrations 2. Surface depletion Deep enrichment 3. DIC < Alk Q? 4. DDIC > DAlk Controls on Ocean Distributions A) Photosynthesis/Respiration Organic matter (approximated as CH2O for this example) is produced and consumed as follows: CH2O + O2 CO2 + H2O Then: CO2 + H2O H2CO3* H2CO3* H+ + HCO3HCO3- H+ + CO32As CO2 is produced during respiration we should observe: pH DIC Alk PCO2 CO2 is an acid The trends will be the opposite for photosynthesis. B) CaCO3 dissolution/precipitation CaCO3(s) Ca2+ + CO3 2Also written as: CO32- is a base CaCO3(s) + CO2 + H2O Ca2+ + 2 HCO3As CaCO3(s) dissolves, CO32- is added to solution. We should observe: pH DIC Alk PCO2 Summary: DIC is from both organic matter and CaCO3 Alk is only from CaCO3 Influence of Nitrogen Uptake/Remineralization on Alkalinity NO3- assimilation by phytoplankton 106 CO2 + 138 H2O + 16 NO3- → (CH2O)106(NH3)16 + 16 OH- + 138 O2 NH4 assimilation by phytoplankton 106 CO2 + 106 H2O + 16 NH4+ → (CH2O)106(NH3)16 + 16 H+ + 106 O2 NO3- uptake is balanced by OH- production Alk ↑ NH4+ uptake leads to H+ generation Alk ↓ Alk = HCO3- + 2 CO32- + OH- - H+ See Brewer and Goldman (1976) L&O Goldman and Brewer (1980) L&O Experimental Culture Ocean Distributions of, DIC, Alk, O2 and PO4 versus Depth and Ocean The main features are: 1. uniform surface values 2. increase with depth 3. Deep ocean values increase from the Atlantic to the Pacific 4. DIC < Alk DDIC > DAlk 5. Profile of pH is similar in shape to O2. 6. Profile of PCO2 (not shown) mirrors O2. Inter-Ocean Comparison Carbonate ion (CO32-) and pH decrease from Atlantic to Pacific x 10-3 mol kg-1 x 10-6 mol kg-1 S = 35 T = 25C Alk 2.300 DIC 1.950 CO32246 pH 8.12 North Atlantic Deep Water 2.350 2.190 128 7.75 Antarctic Deep Water 2.390 2.280 101 7.63 Deep Atlantic to Deep Pacific DAlk = 0.070 DDIC = 0.180 7.46 So DAlk/DDIC = 0.40 Surface Water North Pacific Deep water 2.420 Q? CO2Sys/CO2Calc 2.370 72 CO32- decreases from surface to deep Atlantic to deep Pacific. These CO32- are from CO2Sys. Can Approximate as CO32- ≈ Alk - DIC Composition of Sinking Particles and Predicted Changes Composition of Sinking Particles and Predicted Changes Assume the following average elemental composition of marine particulate matter P N C Ca Si Soft Parts 1 15 105 0 0 Hard Parts 0 0 26 26 50 Composite 1 15 132 26 50 Implies Org C / CaCO3 ~ 105/26 ~4/1 The impact of this material dissolving CH2O + O2 CO2 + H2O DDIC = 1 DAlk = 0 CaCO3 Ca2+ + CO32DDIC = 1 DAlk = 2 DDIC DCa Dalk 1 mol CaCO3 1 1 2 4 mol orgC 4 0 0 Composite 5 1 2 Consequences: 1) DAlk/DDIC = 2/5 = 0.40 (DIC changes more than Alk) 2) Dalk – DDIC ~ DCO32- = 2 – 5 = -3 (CO3 2- decreases) Ocean Alkalinity versus Total CO2 in the Ocean (Broecker and Peng, 1982) DDIC/DAlk ≈ 1.5/1 Work Backwards DAlk / DDIC ≈ 0.66 = 2/3 = 2 mol Org C / 1 mol CaCO3 Emerson and Hedges Color Plate What is composition of sinking particles? Data from annual sediment traps deployments 5 g POC g m-2 y-1 / 12 g mol-1 = 0.42 mol C m-2 y-1 40 g CaCO3 g m-2 y-1 / 105 g mol-1 = 0.38 mol C m-2 y-1 Org C / CaCO3 ~ 1.1 Q. What does this imply? From Klaas and Archer (2002) GBC PIC/POC in sediment trap samples POC and CaCO3 Export Fluxes This Study Previous Studies POC (Gt a−1) Global export 9.6 ± 3.6 11.1–12.9 [Laws et al., 2000]b 9.2 [Aumont et al., 2003]c 8.6 [Heinze et al., 2003]c 8.7–10.0 [Gnanadesikan et al., 2004]c 9.6 [Schlitzer, 2004]d 5.8–6.6 [Moore et al., 2004]c CaCO3 (GtC a−1) Global export 0.52 ± 0.15 0.9–1.1 [Lee, 2001]b 1.8 [Heinze et al., 1999]c 1.64 [Heinze et al., 2003]c 0.68–0.78 [Gnanadesikan et al., 2004]c 0.38 [Moore et al., 2004]c 0.84 [Jin et al., 2006]c 0.5–4.7 [Berelson et al., 2007]b POC/CaCO3 = 9.6 / 0.52 = 18.5 Based on Global Model results of Sarmiento et al (2992) GBC; Dunne et al (2007) GBC Revelle Factor The Revelle buffer factor defines how much CO2 can be absorbed by homogeneous reaction with seawater. B = dPCO2/PCO2 / dDIC/ DIC B = CT / PCO2 (∂PCO2/∂CT)alk = CT (∂PCO2/∂H)alk PCO2 (∂CT/∂H)alk After substitution B ≈ CT / (H2CO3 + CO32-) For typical seawater with pH = 8, Alk = 10-2.7 and CT = 10-2.7 H2CO3 = 10-4.7 and CO32- = 10-3.8; then B = 11.2 Field data from GEOSECS Sundquist et al., Science (1979) dPCO2/PCO2 = B dDIC/DIC A value of 10 tells you that a change of 10% in atm CO2 is required to produce a 1% change in total CO2 content of seawater, By this mechanism the oceans can absorb about half of the increase in atmospheric CO2 B↑ as T↓ as CT↑ Revelle Factor Numerical Example (using CO2Sys) CO2 + CO32- = HCO3- CO2 350ppm + 10% = 385ppm Atm Ocn CO2 → H2CO3 → HCO3- → CO32- at constant alkalinity DIC 11.3 mM 1640.5 mM 183.7 1837 +1.2 (10.6%) +27.7 (1.7%) -11.1 (-6.0%) +17.9 (+0.97%) 12.5 1668.2 174.2 1854.9 The total increase in DIC of +17.9 mM is mostly due to a big change in HCO3- (+27.7 mM) countering a decrease in CO32- (-11.1 mM). Most of the CO2 added to the ocean reacts with CO32- to make HCO3-. The final increase in H2CO3 is a small (+1.2 mM) portion of the total. Emerson and Hedges Plate 8 Effect of El Nino on ∆pCO2 fields High resolution pCO2 measurements in the Pacific since Eq. Pac-92 Eq Pac-92 process study PCO2sw Always greater than atmospheric El Nino Index Cosca et al. in press Photosynthesis/respiration (shown as apparent oxygen utilization or AOU = O2,sat – O2,obs) and CaCO3 dissolution/precipitation vectors (from Park, 1969) CH2O + O2 → CO2 + H2O as O2↓ AOU ↑ CO2 ↑