01_17_18
advertisement

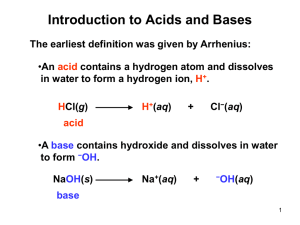
Acids and Bases 3 Boon Chemistry January 17 & 18, 2013 Catalyst Take out your homework please. Label the acid, base, conjugate acid and conjugate base in the following reaction. Draw an arrow showing the movement of a proton from the acid to base. H2CO3 + NH3 HCO3- + NH4+ Objectives I can explain the relationship between pH and H3O+ concentration. I can categorize acids and bases using the pH scale. Agenda Use the definition of acid, base, and conjugate acid and base to justify your answers. Catalyst and Homework Review pH scale Foldable and White Board Practice Practice: pH WS Exit Slip: pH and strong/weak acid/base Exit Slip: Answers Standard 5a The neutralization of any strong acid and strong base produces mostly (a) H2O molecules (c) H3O+ and OH- ions (b) H3O+ ions (d) OH- ions Why? Example: NaOH + HCl NaCl + H2O This was the catalyst Wednesday. A property listed below that is not characteristic of an acid is (a) a sour taste. (c) the ability to conduct an electric current. (b) a slippery feel. (d) reactivity with metals. Why? This is a property of a base. This is in your notes. Exit Slip answers continued Both acids and bases are electrolytes. This means… (a) they are the main ingredients in sports drinks. (b) they produce electricity and will shock you. (c) they conduct electricity when dissolved in water. (d) none of the above. Why? This is the definition of electrolyte. List two examples of acids and two examples of bases that we may find at home. Acids: lemons, tomatoes, coffee, orange juice, stomach acid, etc Bases: soap, cleaning products with ammonia, baking soda, antacid, drain cleaner etc. Exit Slip Review Standard 5b Pure ammonia that is not in aqueous solution does not form hydroxide ions. Why is pure ammonia considered a base using the BrØnsted-Lowry definition? (a) The ammonia molecule accepts protons from other molecules. (b) The ammonia molecule donates protons to other molecules. (c) The ammonia molecule reacts with sulfuric acid. (d) The ammonia molecule donates a hydroxide group to other molecules. Why? Bases are proton acceptors. They gain or take a proton. A substance that produces hydrogen ions (H+) when dissolved in water is classified as (a) an acid. (c) a salt. (b) a base. (d) an electrolyte. Why? Acids are proton (H+) donors or givers or producers. Exit Slip Review Continued In the reaction H3O+ + CO32– → HCO3– + H2O, which compound acts as the base? (a) H3O+ (c) HCO3– (b) CO32– (d) H2O Why? This compound takes a H+ from the H3O+ to form HCO3– . Substances that take protons are bases. The conjugate acid of the chloride ion, Cl-, is (a) Cl2 (c) H+ (b) HCl (d) ClO Why? A conjugate acid is formed by adding a proton (H+). Cl- + H+ → HCl Homework Review page 566 #1, 4, 5, 18, 19, 21 1. A strong acid dissociates completely in solution. A weak acid dissociates only to a small extent in solution. (See pp. 532) 4. The ammonium ion NH4+ is the conjugate acid to the base ammonia (NH3). (See pp. 537.) 5. Water is considered amphoteric because it can act either as an acid, donating a proton, or as a base, accepting a proton. (See pp. 534 for examples of water acting as a base and pp. 536 for examples of water acting as an acid.) Homework Review pp. 538 # 1, 3, 5, 6, 7, 9, 13 18. Weak acids and weak bases are poor electrical conductors or electrolytes because they do not fully dissociate in solution. Therefore, there are not many ions in solution that will conduct electricity. (See pp. 531-532, 534.) 19. The strength of an acid is a measure of whether or not the acid fully dissociates in solution. Strong acids fully dissociate and weak acids do not. The concentration of an acid is a measure of the amount of acid in a certain volume of solution. (See pp. 532 and notes on concentration.) 21. HCN + H2O CN- + H3O+ acid base conjugate conjugate base acid pH Foldable The pH scale: pH is a value used to express the acidity or alkalinity of a solution. pH stands for “power of hydrogen.” The pH scale is a negative logarithmic scale. Alkaline This means that a low pH reflects means base. a high hydronium ion concentration. How does pH and [H3O+] relate to pOH and [OH]? When the concentration of hydronium ions increases, the concentration of hydroxide ions decreases. In fact, if we know the pH or the [H3O+] of a solution, we can determine the pOH and the [OH-]. Here’s how: pH + pOH = 14 [OH-][H3O+] = 10-14 pH + pOH = 14 More Acidic [OH-][H3O+] = 10-14 More Basic 1. The color of a solution identifies if it is an acid, base, or neutral solution. A. True B. False C. Pink are base and clear are acid 2. Which solution is basic? A B D. More than one C E. None 3. Which solution is acidic? A B C D. More than one E. Difficult to tell 4. Which solution is basic? A B D. More than one C E. None 5. Which solution is acidic? A B D. More than one C E. None 6. How will adding water effect the pH? Increase the pH B. Decrease the pH C. No pH change A. A: more water lessens the acidity, so pH goes up 7. How will equal amount of water effect the pH? A. B. C. D. Increase the pH Decrease the pH The pH will be cut in half No pH change B: more water lessens the basicity , so pH goes down, from 10 to 9.7, but not by 2 (log scale) 8. What is the order from most acidic to most basic? A A. A B C B. A C B C.B A C D.C B A E. C A B B C 9. What is the order from most acidic to most basic? A. A B C B. A C B C.B A C D.C B A E. C A B A B C pH Foldable: Back Left of WS Calculate pH from [H3O+] pH = -log [H3O+] Ex: What is the pH of a 0.0001 M HCl solution? Step 1: Convert to scientific notation 0.0001 = 1.0 x 10-4 Step 2: Use the pH equation and your pH scale pH = -log [1.0 x 10-4] = - (-4) =4 pH Foldable: Back Middle of WS Calculate pH from pOH pH + pOH = 14 Ex: What is the pH of a solution of ammonia with a pOH of 5? Step 1: plug the given into the equation. pH + 5 = 14 Step 2: Solve for the unknown pH + 5 = 14 pH + 5 -5 = 14 -5 pH = 9 pH Foldable: Back Right of WS Calculate [H3O+] from pH [H3O+] = 10-pH Ex: What is the hydronium ion concentration of a solution with a pH of 3? Step 1: plug the given into the equation. [H3O+] = 10-3 Step 2: Convert to scientific notation. 10-3 = 1.0 x 10-3 Independent Work Time Expectations: You may work on the following: You must work at your seat. You may speak quietly to the students next to you. Raise your hand if you need help. You may get up to check your answers. pH foldable worksheet pH and pOH worksheet Any other Acid/Base WS or HW Use pp. 530547 for help! Answers are posted at the front of the class. Correct your work. Exit Slip Expectations: Tools: You may use all your notes, worksheets, and flash cards. You may use your own calculator. What do I turn in? You will work silently and independently. When you are done, cover your exit slip with your handouts. You will turn in your exit slip only. Homework: Read pp. 539-547 pp.547 #1-5, pp. 567 # 24, 25, 26 Complete any worksheets or article questions that you have not finished. Homework Due Next Class: Read pp. 539-547 pp.547 #1-5, pp. 567 # 24, 25, 26 Due Next Thursday/Friday: Complete any worksheets or article questions that you have not finished. Practice your vocabulary with flashcards!