Element cards for Periodic Challenge
advertisement
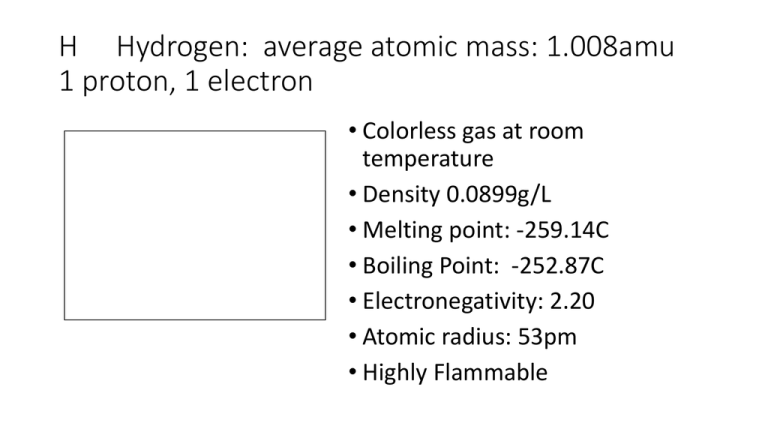
H Hydrogen: average atomic mass: 1.008amu 1 proton, 1 electron • Colorless gas at room temperature • Density 0.0899g/L • Melting point: -259.14C • Boiling Point: -252.87C • Electronegativity: 2.20 • Atomic radius: 53pm • Highly Flammable He Helium: average atomic mass: 4.003amu 2 protons, 2 electrons • Colorless gas at room temperature • Density 0.1785g/L • Melting point: N/A • Boiling Point: -268.93C • Electronegativity: 0 • Atomic radius: 31pm • Very Unreactive Li Lithium: average atomic mass:6.941amu 3 protons, 3 electrons • Light, soft, white/silver shiny metal • Density: 0.535g/mL • Melting point: 180.54C • Boiling Point: 1342C • Electronegativity: 0.98 • Atomic radius: 167pm • Reacts with water to make hydrogen gas Be Beryllium: average atomic mass:9.012amu 4 protons, 4 electrons • Density 1.848g/mL • Melting point: 1287C • Boiling Point: 2470C • Electronegativity: 1.57 • Atomic radius: 112pm • Expensive and toxic • Shiny silvery metal B Boron: average atomic mass:10.81amu 5 protons, 5 electrons • Density 2.460g/mL • Melting point: 2075C • Boiling Point: 4000C • Electronegativity: 2.04 • Atomic radius: 87pm • Hard, brittle sparkly metal C Carbon: average atomic mass: 12.011amu 6 protons, 6 electrons • Density 2.260g/mL • Melting point: 3550C • Boiling Point: 4027C • Electronegativity: 2.55 • Atomic radius: 67pm • Graphite = dark grey matte solid • Diamond = sparkly colorless gemstone N Nitrogen: average atomic mass: 14.007amu 7 protons, 7 electrons • Density 1.251g/L • Melting point: -210.1C • Boiling Point: -195.79C • Electronegativity: 3.04 • Atomic radius: 56pm • Elements always found with 2 atoms bonded together • Colorless gas at room temperature • Makes up 78% of earth’s atmosphere O Oxygen: average atomic mass: 15.999amu 8 protons, 8 electrons • Density 1.429g/L • Melting point: -218.3C • Boiling Point: -182.9C • Electronegativity: 3.44 • Atomic radius: 48pm • Colorless gas at room temperature • Always found with 2 atoms bonded together • 21% of earth’s atmosphere F Fluorine: average atomic mass: 18.998 9 protons, 9 electrons • Density 1.696g/L • Melting point: -219.6C • Boiling Point: -188.12C • Electronegativity 3.98 • Atomic radius 42pm • Pale yellow gas at room temperature • Always found with 2 atoms bonded together • Reacts violently with almost anything Ne Neon: average atomic mass: 20.180amu 10 protons, 10 electrons • Density 0.900g/L • Melting point: -248.59 • Boiling Point: -246.08 • Electronegativity 0 • Atomic radius 38pm • Colorless gas at room temperature • Very unreactive Na Sodium: average atomic mass: 22.990amu 11 protons, 11 electrons • Density 0.968g/mL • Melting point: 97.72C • Boiling Point: 883C • Electronegativity: 0.93 • Atomic radius: 190pm • Soft, malleable silver/white metal • Reacts with water to make hydrogen gas • Reacts with air to form Na2O Mg Magnesium: average atomic mass: 24.305amu 12 protons, 12 electrons • Density 1.738g/mL • Melting point: 650C • Boiling Point: 1090C • Electronegativity 1.31 • Atomic radius 145pm • Lightweight metal • Lights on fire easily • Silvery metal Al Aluminum: average atomic mass: 26.982amu 13 protons, 13 electrons • Density 2.7g/mL • Melting point: 660C • Boiling Point: 2519C • Electronegativity 1.61 • Atomic radius 118pm • Silvery, meltable metal • Poor heat conductor Si Silicon: average atomic mass: 28.085amu 14 protons, 14 electrons • Density 2.330g/mL • Melting point: 1414C • Boiling Point: 2900C • Electronegativity 1.90 • Atomic radius 111pm • Good for semiconductors • Metallic yet crystalline P Phosphorus: average atomic mass: 30.974amu 15 protons, 15 electrons • Density 1.823g/mL • Melting point: 44.2C • Boiling Point: 280.5C • Electronegativity 2.19 • Atomic radius 98pm • Occurs as white, red, and black powders • Highly reactive! Can explode or combust S Sulfur: average atomic mass: 32.065amu 16 protons, 16 electrons • Density 1.960g/mL • Melting point: 115.21C • Boiling Point: 444.72C • Electronegativity 2.58 • Atomic radius 88pm • Elemental form found in nature • Yellow crystals, very pungent Cl Chlorine: average atomic mass: 35.453amu 17 protons, 17 electrons • • • • • • Density 35.453amu Melting point: -101.5C Boiling Point:-34.04C Electronegativity 3.16 Atomic radius 79pm Poisonous yellow-green gas at room temperature • Highly reactive, bonds with Na to make NaCl • Elements always found with 2 atoms bonded together Ar Argon: average atomic mass: 39.948amu 18 protons, 18 electrons • Density 1.784g/L • Melting point: -189.3C • Boiling Point: -185.8C • Electronegativity 0 • Atomic radius 71pm • Colorless gas at room temperature • Extremely unreactive P Potassium: average atomic mass: 39.098amu 19 protons, 19 electrons • Density 0.856g/mL • Melting point: 63.38C • Boiling Point: 759C • Electronegativity 0.82 • Atomic radius 243pm • Silvery metal • Explodes when placed in water • Oxidizes rapidly in air Ca Calcium: average atomic mass: 40.078amu 20 protons, 20 electrons • Density 1.550g/mL • Melting point: 842C • Boiling Point: 1484C • Electronegativity 1.0 • Atomic radius 194pm • Firm silvery metal • Reacts with water to make hydrogen gas • Rarely seen in nature Sc Scandium: average atomic mass: 44.956amu 21 protons, 21 electrons • Density 2.985g/mL • Melting point: 1541C • Boiling Point: 2830C • Electronegativity 1.36 • Atomic radius 184pm • Metallic crystals Ti Titanium: average atomic mass: 47.867amu 22 protons, 22 electrons • Density 4.507g/mL • Melting point: 1668C • Boiling Point: 3287C • Electronegativity 1.54 • Atomic radius 176pm • Light-weight and very strong metal V Vanadium: average atomic mass: 50.942amu 23 protons, 23 electrons • Density 6.110g/mL • Melting point: 1910 C • Boiling Point: 3407C • Electronegativity 1.63 • Atomic radius 171pm • Has several oxidation states • Silver metal Cr Chromium: average atomic mass: 51.996amu 24 protons, 24 electrons • Density 7.140g/mL • Melting point: 1907C • Boiling Point: 2671C • Electronegativity 1.66 • Atomic radius 166pm • Used to plate other things • Very shiny, smooth metal Mn Manganese- average atomic mass:54.938amu 25 protons, 25 electrons • Density 7.470g/mL • Melting point: 1246C • Boiling Point: 2061C • Electronegativity 1.55 • Atomic radius 161pm • Silvery metal Fe Iron: average atomic mass: 55.845amu 26 protons, 26 electrons • Density 7.874g/mL • Melting point: 1538C • Boiling Point: 2861C • Electronegativity 1.83 • Atomic radius 156pm • Soft, silvery metal • most common element on earth by mass Co Cobalt: average atomic mass: 58.933amu 27 protons, 27 electrons • Density 8.9g/mL • Melting point: 1495C • Boiling Point: 2927C • Electronegativity 1.88 • Atomic radius 152pm • Silvery metal Ni Nickel: average atomic mass: 58.693amu 28 protons, 28 electrons • Density 8.908g/mL • Melting point: 1455C • Boiling Point: 2913C • Electronegativity 1.91 • Atomic radius 149pm • Commonly electroplated metal • Hard • Silvery white color Cu Copper: average atomic mass: 63.546amu 29 protons, 29 electrons • Density 8.920g/mL • Melting point: 1084.62C • Boiling Point: 2927C • Electronegativity 1.90 • Atomic radius 145pm • Copper-colored metal • Highly conductive for heat, electricity • Stolen from construction sites Zn Zinc: average atomic mass: 65.39amu 30 protons, 30 electrons • Density 7.140g/mL • Melting point: 419.53C • Boiling Point: 907C • Electronegativity 1.65 • Atomic radius 142pm • More reactive than iron Ga Gallium: average atomic mass: 69.723amu 31 protons, 31 electrons • Density 5.904g/mL • Melting point: 29.76C • Boiling Point: 2204C • Electronegativity 1.81 • Atomic radius 136pm • Metal with low melting point Ge Germanium: average atomic mass: 72.64amu 32 protons, 32 electrons • Density 5.323g/mL • Melting point: 938.3C • Boiling Point: 2820C • Electronegativity 2.01 • Atomic radius 125pm • Used in semiconductors and diodes • Metallic with internal crystal structure As Arsenic: average atomic mass: 74.922amu 33 protons, 33 electrons • Density 5.727g/mL • Melting point: 817C • Boiling Point:614C • Electronegativity 2.18 • Atomic radius 114pm • crystalline Se Selenium: average atomic mass: 78.96amu 34 protons, 34 electrons • Density 4.819g/mL • Melting point: 221C • Boiling Point: 685C • Electronegativity 2.55 • Atomic radius 103pm • Found in pure form in nature • Used in light sensing applications Br Bromine: average atomic mass: 79.904amu 35 protons, 35 electrons • Density 3.120g/mL • Melting point: -7.3C • Boiling Point: 59C • Electronegativity 2.96 • Atomic radius 94pm • Brown liquid at room temp • Always found with 2 atoms bonded together • Very toxic and reactive Kr Krypton: average atomic mass: 83.8amu 36 protons, 36 electrons • Density 3.75g/L • Melting point: -157C • Boiling Point: -153C • Electronegativity 3.0 • Atomic radius 88pm • Non-reactive • Colorless gas Rb Rubidium: average atomic mass: 85.468amu 37 protons, 37 electrons • Density 1.532g/mL • Melting point: 39.31C • Boiling Point: 688C • Electronegativity 0.82 • Atomic radius 265pm • Highly reactive in air • Explosive in water • Silvery metal Sr Strontium: average atomic mass: 87.62amu 38 protons, 38 electrons • Density 2.630 g/mL • Melting point: 777C • Boiling Point: 1382C • Electronegativity 0.95 • Atomic radius 219pm • Soft, silvery-white metal Y Yttrium: average atomic mass: 88.906amu 39 protons, 39 electrons • Density 4.472g/mL • Melting point: 1526C • Boiling Point: 3345C • Electronegativity 1.22 • Atomic radius 212pm • Rarely found pure Zr Zirconium: average atomic mass: 91.224amu 40 protons, 40 electrons • Density 6.511g/mL • Melting point: 1855C • Boiling Point: 4409C • Electronegativity 1.33 • Atomic radius 206pm • Silvery metal • Used in jewelry and nuclear industry Nb Niobium: average atomic mass: 92.906amu 41 protons, 41 electrons • Density 8.570g/mL • Melting point: 2477C • Boiling Point: 4744C • Electronegativity 1.6 • Atomic radius 198pm • Used in jewelry Mo Molybdenum: average atomic mass: 95.94amu 42 protons, 42 electrons • Density 10.280g/mL • Melting point: 2623C • Boiling Point: 4639C • Electronegativity 2.16 • Atomic radius 190pm • Used in high temperature applications –maintains strength Tc Technetium: average atomic mass: 98amu 43 protons, 43 electrons • Density 11.5g/mL • Melting point: 2157C • Boiling Point: 4265C • Electronegativity 1.9 • Atomic radius 183pm • Radioactive • Used in skeleton imaging Ru Ruthenium: average atomic mass: 101.07amu 44 protons, 44 electrons • Density 12.370g/mL • Melting point: 2334C • Boiling Point: 4150C • Electronegativity 2.2 • Atomic radius 178pm • Used in jewelry Rh Rhodium: average atomic mass: 102.91amu 45 protons, 45 electrons • Density 12.450g/mL • Melting point: 1964C • Boiling Point: 3695C • Electronegativity 2.28 • Atomic radius 173pm • Super shiny metal used to plate jewelry • Expensive metal Pd Palladium: average atomic mass: 106.42amu 46 protons, 46 electrons • Density 12.033g/mL • Melting point: 1554.9C • Boiling Point: 2963C • Electronegativity 2.20 • Atomic radius 169pm • Unreactive in air • Silver-colored metal • Used in jewelry Ag Silver: average atomic mass: 107.87amu 47 protons, 47 electrons • Density 10.490g/mL • Melting point: 961.78C • Boiling Point: 2162C • Electronegativity 1.93 • Atomic radius 165pm • Used in coins, jewelry Cd Cadmium: average atomic mass: 112.411amu 48 protons, 48 electrons • Density 8.650g/mL • Melting point: 321.07C • Boiling Point: 767C • Electronegativity 1.69 • Atomic radius 161pm • metal In Indium: average atomic mass: 114.818amu 49 protons, 49 electrons • Density 7.310g/mL • Melting point: 156.6C • Boiling Point: 2072C • Electronegativity 1.78 • Atomic radius 156pm • Used commercially in alloys Sn Tin: average atomic mass: 118.710amu 50 protons, 50 electrons • Density 7.310g/mL • Melting point: 231.93C • Boiling Point: 2602C • Electronegativity 1.96 • Atomic radius 145pm • metal Sb Antimony: average atomic mass: 121.76amu 51 protons, 51 electrons • Density 6.697g/mL • Melting point: 630.63C • Boiling Point: 1587C • Electronegativity 2.05 • Atomic radius 133pm • Sparkling broken crystals • Commonly in alloys to make bullets and batteries Te Tellurium: average atomic mass: 127.6amu 52 protons, 52 electrons • Density 6.240g/mL • Melting point: 449.51C • Boiling Point: 988C • Electronegativity 2.1 • Atomic radius 123pm I Iodine: average atomic mass: 126.90amu 53 protons, 53 electrons • Density 4.940g/mL • Melting point: 113.7C • Boiling Point: 184.3C • Electronegativity 2.66 • Atomic radius 115pm • Solid at room temperature • Bluish-black • Usually found with 2 bonded together Xe Xenon: average atomic mass: 131.29amu 54 protons, 54 electrons • Density 5.9g/L • Melting point: -111.8C • Boiling Point: -108C • Electronegativity 2.6 • Atomic radius 108pm • Colorless, dense gas