Hypersonic Fuels Chemistry: n-Heptane Cracking and Combustion
advertisement

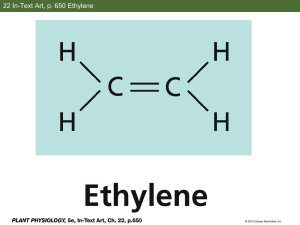
Hypersonic Fuels Chemistry: n-Heptane Cracking and Combustion Andrew Mandelbaum - Dept. of Mechanical Engineering, Princeton University Alex Fridlyand - Dept. of Mechanical Engineering, University of Illinois at Chicago Prof. Kenneth Brezinsky - Dept. of Mechanical Engineering, University of Illinois at Chicago Outline • • • • Project Background Hypothesis Experimental Apparatus and Methods Results and Modeling ▫ Heptane Pyrolysis ▫ Heptane Oxidation ▫ Heptane/Ethylene Oxidation • Conclusions Project Background Fig. 1: Cross-sectional diagram of a scramjet engine1 • Heat management • Very short reaction time requirements 1. How Scramjets Work [online]. NASA. 2 Sept. 2006. 4 June 2011. http://www.nasa.gov/centers/langley/news/factsheets/X43A_2006_5.html. Project Background • Use fuel to cool engine structure • Shorter cracking products may ignite more readily Fig. 2: Ignition delay vs. temperature for various pure gases and mixtures2 2. M. Colket, III and L. Spadaccini: Journal of Propulsion and Power, 2001, 17.2, 319. Consequence, Questions Raised, Applications • Injected fuel – different from fuel in tank • Effect on combustion products? • What causes the change in energy output – physical or chemical differences? • Improved chemical simulations ▫ Improved accuracy ▫ Use in engine modeling software ▫ Possibility for fuel composition customization Hypothesis • Heptane cracking products (primarily ethylene) will chemically influence combustion of remaining fuel • Resultant species - differ in from non-cracked fuel alone and from existing heptane models Low Pressure Shock Tube Fig. 3: Schematic drawing of low pressure shock tube and related assemblies • Designed to operate from 0.1-10 bar, 800-3000 K, 1-3 ms reaction time • Explore oxidation chemistry at pressures relevant to hypersonic engine combustor Methods • Perform pyrolysis and oxidation shocks at 4 bar driver pressure • Examine stable intermediates and fuel decay process using gas chromatography (GC-FID/TCD) • Model used: n-Heptane Mechanism v3, Westbrook et al3, 4, 5 • Note: all graphs have x-error of ±5-10 K (from pressure transducers) and y-error of ±5-10% (from standards used in calibrations and GC error). Error bars are omitted for clarity 3. Mehl, M., H.J. Curran, W.J. Pitz and C.K. Westbrook: "Chemical kinetic modeling of component mixtures relevant to gasoline," European Combustion Meeting, 2009. 4. Mehl, M., W.J. Pitz, M. Sjöberg and J.E. Dec: “Detailed kinetic modeling of low-temperature heat release for PRF fuels in an HCCI engine,” S AE 2009 International Powertrains, Fuels and Lubricants Meeting, SAE Paper No. 2009-01-1806, Florence, Italy, 2009. 5. Curran, H. J., P. Gaffuri, W. J. Pitz, and C. K. Westbrook: Combustion and Flame,1998, 114, 149-177 Heptane Pyrolysis Heptane Decomposition - Pyrolysis 60 Heptane Concentration [ppm] 50 Pdriver=4 bar Rxn time: 1.51.8 ms 40 30 20 10 0 900 950 1000 1050 1100 1150 1200 1250 1300 1350 1400 1450 T5 Calibrated [K] Fig. 4: Concentration of heptane vs. T5 during pyrolysis • Pyrolyze to characterize decomposition and species formed Heptane Pyrolysis (Continued) Ethylene Concentration - Pyrolysis 140 Ethylene Concentration [ppm] 120 Pdriver=4 bar Rxn time: 1.51.8 ms 100 80 60 40 20 0 900 950 1000 1050 1100 1150 1200 1250 1300 1350 1400 1450 T5 Calibrated [K] Fig. 5: Concentration of ethylene vs. T5 during pyrolysis • Ethylene is the primary product by concentration Heptane Pyrolysis (Continued) Acetylene, Methane, and Propylene Concentration 70 Acetylene Methane Propylene Concentration [ppm] 60 50 40 30 20 10 0 900 950 1000 1050 1100 1150 1200 1250 1300 1350 1400 1450 T5 Calibrated [K] Fig. 6: Concentration of acetylene, methane, and propylene vs. T5 during pyrolysis • Possible directions for future research Heptane Pyrolysis - Modeling Heptane Concentration vs. Final Temperature 60 Heptane Pyrolysis (Data) Heptane Pyrolysis (Model) 50 Pdriver=4 bar Rxn time: 1.51.8 ms Concentration [ppm] 40 30 20 10 0 -10 950 1000 1050 1100 1150 1200 1250 1300 1350 1400 T5 [K] Fig. 7: Comparison of pyrolysis data to model results for heptane decomposition • Model results to validate shock tube operation Heptane Oxidation – Modeling and Data Oxygen Concentration vs. Final Temperature 450 Pdriver=4 bar Rxn time: 1.51.8 ms Φ=1.38 400 Concentration [ppm] 350 300 250 200 150 100 O2 Concentration (Data) O2 Concentration (Model) 50 0 900 950 1000 1050 1100 1150 1200 1250 1300 1350 1400 T5 [K] Fig. 8: Comparison of oxidation data to model results for oxygen concentration Heptane Oxidation – Modeling and Data (Cont’d) Pdriver=4 bar Rxn time: 1.51.8 ms Φ=1.38 Ethylene Concentration vs. Final Temperature 120 Ethylene Production (Data) Ethylene Production (Model) Concentration [ppm] 100 80 60 40 20 0 900 950 1000 1050 1100 1150 1200 1250 1300 1350 1400 1450 T5 [K] Fig. 9: Comparison of oxidation data to model results for ethylene concentration Heptane Oxidation – Modeling and Data (Cont’d) Pdriver=4 bar Rxn time: 1.51.8 ms Φ=1.38 Carbon Monoxide Concentration vs. Final Temperature 250 CO Production (Data) CO Production (Model) Concentration [ppm] 200 150 100 50 0 -50 900 950 1000 1050 1100 1150 1200 1250 1300 1350 1400 T5 [K] Fig. 10: Comparison of oxidation data to model results for carbon monoxide production Heptane with Ethylene Oxidation Normalized Heptane Decomposition - Neat vs. Cracked Mixture Normalized Ethylene Concentration - Neat vs. Cracked Micture 2.5 Cracked Fuel Mix Neat Heptane 2 Ethylene Concentration [ppm] Heptane Concentration [ppm] 1 Neat Heptane (Data) Cracked Fuel Mix (Data) Neat Heptane (Model) Cracked Fuel Mix (Model) 0.8 0.6 0.4 1.5 1 0.5 0.2 0 900 950 1000 1050 1100 1150 1200 1250 1300 1350 1400 1450 T5 Calibrated [K] 0 900 950 1000 1050 1100 1150 1200 1250 1300 1350 1400 T5 Calibrated [K] Fig. 11: Normalized heptane concentration and ethylene concentration vs. T5 for neat mixture and cracked fuel mixture 1450 Heptane with Ethylene Oxidation Carbon Monoxide Concentration - Neat vs. Cracked Micture Pdriver=4 bar Rxn time: 1.51.8 ms Φ=1.38 Carbon Monoxide Concentration [ppm] 300 Neat Heptane (Data) Cracked Fuel Mix (Data) Neat Heptane (Model) Cracked Fuel Mix (Model) 250 200 150 100 50 0 -50 900 950 1000 1050 1100 1150 1200 1250 1300 1350 1400 T5 Calibrated [K] Figure 12: Carbon monoxide concentration vs. T5 for pure heptane oxidation and heptane with ethylene Conclusions and Future Work • Heptane cracking products affect combustion of non-cracked fuel through chemical processes • CO, CO2, and H2O production - energy output differences • Future experiments - other cracking products and/or different reaction pressures Acknowledgements • National Science Foundation, EEC-NSF Grant # 1062943 • University of Illinois at Chicago REU • Prof. Christos Takoudis and Dr. Gregory Jursich • Arman Butt and Runshen Xu Questions 6 6. http://www.af.mil/shared/media/photodb/photos/100520-F-9999B-111.jpg Calibrations TFE and CPCN Calibrations for 1, 4, and 10 bar 1550 1450 1350 T5 [K] 1250 1150 1050 950 850 575 625 675 725 775 825 875 W [m/s] Fig. 13: TFE and CPCN shock calibration results • Temperature calibrations using TFE and CPCN • Known decomposition rates allow these species to be used as chemical thermometers Heptane with Ethylene Oxidation (Cont’d) Butene Concentration - Neat vs. Cracked Micture 10 Cracked Fuel Mix Neat Heptane Butene Concentration [ppm] 9 8 7 6 5 4 3 2 1 0 900 950 1000 1050 1100 1150 1200 1250 1300 1350 1400 1450 T5 Calibrated [K] Fig. 14: Butene concentration vs. T5 for neat mixture and cracked fuel mixture Heptane with Ethylene Oxidation (Cont’d) Oxygen Concentration - Neat vs. Cracked Micture 450 Cracked Fuel Mix Neat Heptane 400 Oxygen Concentration [ppm] 350 300 250 200 150 100 50 0 900 950 1000 1050 1100 1150 1200 1250 1300 1350 1400 1450 T5 Calibrated [K] Fig. 15: Oxygen concentration vs. T5 for neat mixture and cracked fuel mixture Heptane w/ Ethylene - Modeling CO Concentration vs. Final Temperature 300 CO Concentration [ppm] 250 Neat CO Production CO Production w/ H2 Balance CO Production w/o H2 Balance 200 150 100 50 0 -50 800 1000 1200 1400 1600 1800 2000 Temperature T5 [K] Fig. 16: Carbon monoxide concentration vs. T5 for neat mixture and mixtures with and without hydrogen balance • Model cracked fuel mix with and without complete hydrogen balance to validate mixture Heptane w/ Ethylene – Modeling (Cont’d) H2O Concentration vs. Final Temperature 350 H2O Concentration [ppm] 300 Neat H2O Production H2O Production w/ H2 Balance H2O Production w/o H2 Balance 250 200 150 100 50 0 -50 800 1000 1200 1400 1600 1800 2000 Temperature T5 [K] Fig. 17: Water concentration vs. T5 for neat mixture and mixtures with and without hydrogen balance • Decreased H2O output without H balance