Team5-Project2-Metha..
advertisement

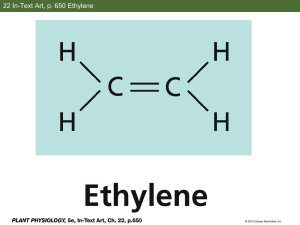
Catalytic Conversion (via Soft Oxidation) of Methane to Ethylene Group 5 Agenda Background Introduction Project Objectives and Production Targets Constraints and Tentative Flow Diagram Safety and Environmental Concerns Market Analysis Introduction Why we are interested in ethylene and its derivatives? Ethylene is the world’s largest commodity chemical and the chemical industry’s fundamental building block. Ethylene and its derivatives applications Introduction Natural gas: is abundant hydrocarbon feedstock. Methane: is the principal component of natural gas, has high hydrogen: carbon ratio. Introduction Current approaches for the direct, large-scale chemical transformation of methane to useful chemicals: aromatization, oxyclorination, and oxidative coupling. Disadvantages: modest selectivity and yield, requirement for corrosive reagents, heat management and temperature control. New approach??? Sulfur: a soft oxidant for conversion of methane to ethylene => Using gaseous sulfur (S2) as a soft oxidant can hinder the over-oxidation of methane when compared with using O2 as oxidant. Project Objectives With feedstock as natural gas that has the same composition in project 1, we study and maximize ethylene production from methane through oxidation conversion. CHEMCAD would be used as design simulator to assert the process and economic feasibility of the project. Production Targets 100 molar basis of feed gas: The highest possible recovery ethylene C2H4. Minimal H2S release to meet environmental regulation. Minimize the formation of undesirable byproducts during the conversion of CH4 to C2H4. High ROI. Constraints Modest selectivities and yields Heat management and temperature control Requirement for corrosive reagents Dependence on highly toxic halogenated intermediates Highly capital-intensive GENERAL STEPS I. Reaction Site Soft oxidation of methane with sulfur II. Purification Site H2S absorption with DEA Regeneration of DEA III. Separation Site CS2 removal CH4 removal (Recycle back to feed) C2H6 removal UNIT OPERATIONS Kinetic Reactor Pumps Heat Exchangers Mixers Absorber Distillation Column TENTATIVE FLOW DIAGRAM Environmental concerns Three main issues considered when dealing with the production of ethylene : Global warming Greenhouse gas effects contributed by CS2 and CH4 Extensive use of land Drilling pads Landscape damage Ethylene environmental hazard: C2H4 can cause damage to plants and materials as a VOC Health Concerns CS2 Hydroca rbons H2S CH3SH C2H4 Potential acute health effects -Irritating to eyes, skin and respiratory system - Harmful if swallowed. -May cause burns or frostbites -Act as a simple asphyxiant Moderately irritating to eyes and skin - May cause burns or frostbites -very toxic to inhalation -Irritating to eyes -May causes severe burns or frostbites -very toxic by inhalation May causes severe burns or frostbites -acts as a simple asphyxiant. Potential chronic health effects Behavioral and neurophysiologic al changes. -reduced nerve conduction velocity. Possible damage to heart and central nervous system Possible damage to lungs, upper respiratory tract, eyes, central nervous system Possible damage to blood, eyes, kidneys, lungs, livers, and upper respiratory tract. Possible damage to lungs,heart, Muscle tissue. Exposure Limits Regulations CS2 Hydrocarbons H2S Permissible Exposure Limits (PEL) by OSHA STEL(1989): -Not available -12 ppm, 15 mins TWA (1989): -12 mg/m³, 8 hrs. STEL (1989) -21mg/m3 , 15 min - 15 ppm, 15 min TWA (2012): -Not -1 mg/m³, available 8 hrs -0.5 ppm, 8 hrs Threshold Limit values (TLV) by ACGIH TWA (2009): -1 ppm, 8 hrs STEL (2010): -5ppm, 15 min TWA (2010) - 1 ppm 8hrs TWA (2012): TWA(2010): -0.98 mg/m³, -200 ppm - 8 hrs. 8 hrs -0.5 ppm 8 hrs TWA(2010) -1000 ppm,8hrs CH3SH OSHA: Occupational safety and Health Administration ACGIH: American Conference of Industrial Hygienists PEL: Permissible Exposure Limit ; STEL: Short-term exposure limit TWA: Time-weighted average C2H4 Safety Precautions HIGH CONTROL SYSTEMS Vibration alarms, toxic gas detectors, combustible gas or fire detectors to potential emergency situations detections Enclose operations and provide local exhaust ventilation at the site of chemical release. Provision of fire protection and emergency facilities by additional facilities for emergency shutdown and isolation. Secondary enclosures (building a vessel around the equipment) for catching leaks for storage or handling of highly toxic materials discharges, or others. Use of respiratory and protective equipment Ethylene, Methanol and Propylene Expanding At A Rapid Pace Ethylene is the largest of the basic chemical building blocks Ethylene, propylene and methanol are expanding at a rapid pace…driven by shale in North America Benzene and chlorine showing more modest growth Ethylene, Methanol and Propylene Expanding At A Rapid Pace 2020 Global Capacity: Ethylene:200 Million Tons Methanol:160 Million Tons Propylene:140 Million Tons Price trend of Ethylene Demand for Basic Chemicals Driven By Durable/Non-durable Goods • Strong economic growth supports basic chemical demand growth • Modest growth in 2012/13 suggesting lower consumer spending • Emerging markets are driving tomorrows demand growth • China dynamics are changing, but remains critical to most markets Ethylene Investments North America Ethylene Capacity Forecast To Reach 45 Million Metric Tons Thank you! QUESTIONS????