Principles of Spectroscopy
Dr. Alison Willows
Created with MindGenius Business 2005®
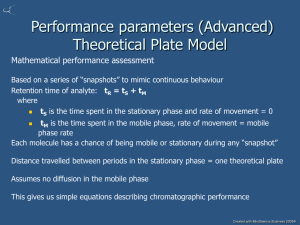
Introduction
Spectral lines occur in spectroscopy through the
absorption, emission or scattering of a photon
when the energy of a molecule or atom
changes.
In atomic spectroscopy the change in energy is
a result of electronic transitions (see CH115)
In molecular spectroscopy the energy of the
molecule also changes due to changes of
rotational and vibrational state
Ultra-violet and visible spectroscopy looks at
the change of electron distribution
Created with MindGenius Business 2005®
Introduction
Molecular spectra thus contain more
information than atomic spectra, e.g.
Bond strengths, lengths and angles and
molecular dimensions, shapes and dipole
moments
These energy changes can be detected
using Infra-red spectroscopy (vibration)
and microwave spectroscopy (rotation)
Created with MindGenius Business 2005®
Electromagnetic spectrum
Created with MindGenius Business 2005®
Introduction
Copyright © Houghton Mifflin Company. All rights reserved.
Created with MindGenius Business 2005®
Infrared spectroscopy
Used to determine the types of bonds
present in a molecule
Infrared radiation is passed through a
sample and the radiation transmitted is
recorded.
Radiation absorbed by the molecule
appears as a band in the spectrum
Instruments scan from around 700 to 5000
cm-1
Created with MindGenius Business 2005®
Created with MindGenius Business 2005®
Infrared activity
Infrared active if normal mode motion is
accompanied by a change of dipole moment
Where normal mode motion is an independent,
synchronous motion of atoms (or groups of
atoms) that may be excited without leading to
excitation of any other normal mode
Created with MindGenius Business 2005®
Infrared spectroscopy
Stretching and bending motions of a CH2 group that
requires energies in the infrared region
Created with MindGenius Business 2005®
Bond stretching frequencies
Created with MindGenius Business 2005®
Bond stretching frequencies, cont.
Vibration of bonds can be described using
Hooke’s law
Frequency of a stretching vibration is directly
proportional to the strength of the bond and
inversely proportional to the masses at either
end
e.g. C-H, N-H and O-H bond stretching
vibrations are high frequency (short
wavelength) compared to those of C-C and C-O
because of low mass of hydrogen compared to
carbon or oxygen
Created with MindGenius Business 2005®
Bond stretching frequencies, cont.
Double bonds is generally stronger and
‘stiffer’ so C=C vibration is at a higher
frequency than C-C
Triple bonds stretch at even higher
frequencies
vibration frequency corresponds to the
relationship of bond strength
Created with MindGenius Business 2005®
Spectra characteristics
Intensity of IR absorption bands is proportional
to the change in dipole moment
Intensity is not proportional to the number of
atoms causing the dipole moments (unlike NMR
where area of peaks is proportional to the
number of hydrogen atoms causing them)
It is not usually possible to assign all of the
peaks in an IR spectrum (unlike NMR)
Created with MindGenius Business 2005®
Spectra characteristics
Peaks with longer wavelengths than 1250
cm-1 are the result of combinations of
vibrations so are characteristic of the
whole molecule rather than a functional
group
This is termed the fingerprint region
No two organic compounds will have the
same IR spectrum, even if the UV and
NMR spectra are the same
Created with MindGenius Business 2005®
Spectra analysis
Not practical to calculate theoretical spectra
Analysis is done by comparison with other
spectra
Many modern instruments come with extensive
libraries of known compounds
The acquired spectrum is compared to those
stored in the library and a list of compounds
with spectra that have matching or similar
absorption signals is generated
For simple molecules it is possible to identify
functional groups by hand
Created with MindGenius Business 2005®
Spectra Analysis, cont.
Pay most attention to the strongest
absorptions
Pay more attention to the peaks to the
left of 1250 cm-1 (shorter wavelengths)
Pay as much attention to the absence of
certain peaks as to the presence of
others
Be wary of O-H peaks as water is a
common contaminant of samples
Created with MindGenius Business 2005®
Spectra
comparison
Infrared spectra of
two similar ketones
Show similar
functional group
bands
Differ in fingerprint
region
Created with MindGenius Business 2005®
Spectra feature summary
Functional group bands will appear in the
same range regardless of details of
molecular structure
Bands in the fingerprint region will be
unique for each compound
Created with MindGenius Business 2005®
Questions
Using IR spectroscopy how could you
quickly distinguish between the structural
isomers benzyl alcohol and anisole?
anisole
Benzyl alcohol’s spectrum will show a
band in the O-H stretching frequency
region (3200 to 3700 cm-1), anisole will
not
Created with MindGenius Business 2005®
These are the infrared spectra of hexanoic acid, 1-pentanol,
cyclohexane and 3-pentanone. Match each compound with the
correct spectrum, indicating which IR bands you used to make your
assignments
Created with MindGenius Business 2005®
Structures
Hexanoic acid
1-pentanol
Cyclohexane
3-pentanone
Created with MindGenius Business 2005®
Fourier Transform Infrared (FTIR)
Computer based: can run multiple scans
increasing signal-to-noise ratio
Faster and more accurate than traditional
double beam instruments
Uses a Michelson Interferometer
Created with MindGenius Business 2005®
FTIR operation
IR beam is split so half goes to a fixed mirror
and half to a moving mirror
The moving mirror changes the phase of the
one beam sinusoidally
The frequency changes as the mirror moves
The beams reinforce or destructively interfere
giving an interferogram
The digitized interferogram is converted to a
function of frequency by a Fourier transform
operation creating a spectrum
Created with MindGenius Business 2005®
FTIR advantages
All IR frequencies are scanned
simultaneously (spectrum obtained in
seconds)
High resolution is possible without losing
signal strength as there is no slit to select
wavelength
Small samples or very dilute solutions can
be analysed as it is possible to sum
hundreds of scans
Created with MindGenius Business 2005®
Rotational Spectra
The energy for small molecules undergoing
rotation is in the region 0.1 – 10 cm-1.
This is in the microwave region of the EM
spectrum
Transitions are detected by monitoring net
absorption of microwave radiation
The rotation of a three dimensional body can
be quite complex.
Rotation is described as components around
three perpendicular axes through the centre of
gravity
Created with MindGenius Business 2005®
Moments of inertia
Rotational properties of a molecule can be
expressed in terms of moments of inertia around
the three perpendicular axes; A, B & C
The moment of inertia, I, of a molecule is defined
as the mass of each atom multiplied by the square
of its distance from the rotational axis through the
centre of the molecule
Where ri is the perpendicular distance
of the atom i from the axis of rotation
Created with MindGenius Business 2005®
Types of rotation
If we regard molecules as rigid motors, bodies
that do not distort under the stress of rotation,
then we can classify four types:
Linear rotors – one moment of inertia equal to zero,
IA = 0,(e.g. CO2, HCl, HCCH)
Symmetric rotors – 2 equal moments of inertia, IB =
IC, IA ≠ 0 (e.g. NH3, CH3Cl and CH3CN)
Asymmetric rotors – 3 different moments of inertia,
IA ≠ IB ≠ IC (e.g. H2O, H2CO and CH3OH)
Spherical rotors – 3 equal moments of inertia, IA = IB
= IC (e.g. CH4, SiH4, SF6)
Created with MindGenius Business 2005®
Four types of rotation
Created with MindGenius Business 2005®
Linear rotors
The atoms in the molecule are arranged
in a straight line
E.g. H — Cl
The three directions of rotation are:
About the bond axis
End-over-end rotation in the plane of the
screen
End-over-end rotation perpendicular to the
screen
Created with MindGenius Business 2005®
Symmetrical rotors
Two of the moments of inertia are equal,
the third does not equal zero
As with linear rotors, the end-over-end
rotations are equal
Two types of symmetrical rotor:
Prolate – IB = IC > IA
Oblate - IB = IC < IA
Created with MindGenius Business 2005®
Asymmetric rotors
Majority of substances belong to this
category
All three moments of inertia are different
IA ≠ IB ≠ IC
E.g. H2O and vinyl chloride CH2 = CHCl
Created with MindGenius Business 2005®
Spherical rotors
All three moments of inertia are equal
Due to their symmetry they have no
dipole moment
Rotation alone can not produce a dipole
so no rotational spectrum is observed
These rotors will not be considered
further
Created with MindGenius Business 2005®
Rotational spectra
Rotational energy is quantized, like all other
forms of molecular energy
Rotational energy levels may be calculated
using the Schrödinger equation for the type of
molecule
Allowed rotational energies of a linear
or spherical rotor
Where J is the angular momentum quantum
number, F(J) is the rotational term (energy)
and B is the rotational constant (cm-1)
When J = 0 the molecule is not rotating
When J = 1 the molecule has its lowest
angular momentum
Selection rule J = 1
J
6
F(J)
42B
5
30B
4
20B
3
12B
2
1
0
6B
2B
0
Created with MindGenius Business 2005®
Linear and spherical motors
The energy of a rotational state is given
by:
F(J) = BJ(J+1)
Where F(J) is the rotational term, a
wavenumber (cm-1), B is the rotational
constant (cm-1) and J is the angular
momentum quantum number
Created with MindGenius Business 2005®
Rotational spectra, cont.
Determining the rotational constant B
allows the bond length to be calculated
So the measurement and identification of
one spectral line allows calculation of
moment of inertia and thus bond length
Created with MindGenius Business 2005®
Moments of
inertia for
different types
of molecules
Created with MindGenius Business 2005®
Points to consider
As the moment of inertia for end-over-end
rotation is greater than that for a diatomic
molecule the B value will be smaller and hence
the spectral lines will be closer together
B values for diatomic molecules are about 10
cm-1.
B values for triatomic molecules can be 1 cm-1
or less and smaller still for larger molecules
The larger the molecule the closer together the
spectral lines
Created with MindGenius Business 2005®
UV-visible Spectroscopy
Absorption of short wavelength, high-energy UV
radiation results in electrons moving from one energy
level to another with energies capable of breaking
chemical bonds
Many molecules will produce only one major absorption
signal
Many combinations of bonded systems absorb at
nearly the same wavelength (C-C, C=O, C=N, triple
bonds and aromatic rings)
Because of this structure is often found using other
techniques and the UV-vis spectrum interpreted based
on this
Created with MindGenius Business 2005®
UV-vis spectroscopy
Visible-ultraviolet spectra are most commonly
used to detect conjugation
Generally, molecules with no double bonds or
with only one double bond do not absorb in the
uv-vis region
Conjugated systems do absorb in this region
The greater the conjugation the longer the
wavelength of max. Absorption
Most of the reliable and useful data is from
strongly absorbing chromophores (>200),
mainly indicative of conjugated or aromatic
systems
Created with MindGenius Business 2005®
Beer’s Law revisited
Absorbance is related to the concentration and
molar absorptivity of a sample
A cl
Where A is the absorbance, is the molar
absorptivity, c is the concentration (mol dm-3),
l is the pathlength of the cell
for any peak in a spectrum is a characteristic
of that particular molecular structure
varies between 10 and 105 for common
chromophores, so tables often give log10 for
manageability
Created with MindGenius Business 2005®
absorption
Created with MindGenius Business 2005®
Classification of UV absorption bands
UV absorption bands have fine structure due to
presence of vibrational sub-levels
However, this is rarely observed in solution due
to collisional broadening
As transitions are associated with changes of
electron orbitals they are often described in
terms of the orbitals involved, e.g.
Where n denotes a non-bonding orbital and
the asterix denotes an antibonding orbital
Created with MindGenius Business 2005®
UV absorption bands, cont.
A molecule can give more than one UV
absorption band, because either
It contains more than one chromophore, or
More than one transition of a single
chromophore is observed
UV spectra contain fewer features than
IR, MS or NMR so less information can be
obtained from them
Created with MindGenius Business 2005®
Important UV chromophores
Dienes
(2 C=C)
and Polyenes
(alternating single and double C bonds)
Extension of conjugation in a carbon chain is
always associated with a shift towards a
longer wavelength, and usually towards
greater intensity
When there are more than 8 conjugated
double bonds max is in the visible region
Woodward’s rules are available to estimate
the position of max
Created with MindGenius Business 2005®
Effect of extended conjugation on UV
absorption
Created with MindGenius Business 2005®
Carbonyl compounds
All carbonyl
derivatives exhibit
weak absorption
although this is not
very useful in
determining structure
Conjugated carbonyl
derivatives always
exhibit strong
absorption (see right)
Created with MindGenius Business 2005®
Benzene derivatives
Exhibit medium to strong absorption in
UV region
Bands usually have characteristic fine
structure
Intensity of absorption strongly
influenced by substituents
Created with MindGenius Business 2005®
UV absorption bands in common
benzene derivatives
Weak auxochromes CH3, -Cl, -OCH3
Groups that increase
conjugation: -CH=CH2,
-C(=O)-R, -NO2
Auxochromes whose
absorption is pH
dependent: -NH2, -OH
Auxochrome is a group of atoms attached to a chromophore
which can increase the wavelength it absorbs light and
increase the intensity
Created with MindGenius Business 2005®
Common solvents for UV-vis
For visible spectroscopy any transparent
solvent can be used
For UV spectroscopy
95% ethanol (absolute ethanol often contains
residual benzene which absorbs in UV)
methanol
water
saturated hydrocarbons such as hexane,
trimethylpentane, isooctane
Created with MindGenius Business 2005®
Sample cells
Care must be taken to select correct cells for
wavelength of interest
For UV work can not use glass as it absorbs UV
radiation; use only for 340 - 1000 nm (visible)
Lower wavelengths (UV) use silica cells (down
to 220 nm) or disposable PMMA (280 - 800 nm)
Below this a special grade of silica is required
(down to 185 nm)
Cells must be very clean (a fingerprint can give
a spectrum of its own)
Created with MindGenius Business 2005®
Fluorescence spectroscopy
Caused by absorption of energy followed by
emission of some of it in the form of light
Stoke’s law tells us the emitted light almost
always has higher wavelength than the
absorbed light
A molecule absorbs a photon of UV radiation
and undergoes a transition to an excited state,
one of its electrons is promoted to an orbital of
higher energy
In fluorescence the excited molecule returns to
the ground state immediately (10-12 to 10-9 s)
Created with MindGenius Business 2005®
Fluorescence, cont.
Two important types of transition for organic
molecules:
n * in which an electron in a non-bonding orbital is
promoted to a -antibonding orbital
* in which an electron in a bonding orbital is raised to a
-antibonding orbital
* leads to significant fluorescence
The extent to which a molecule fluoresces depends on
its structure
In general, intense fluorescence is associated with
molecules that have an extensive system of conjugated
double bonds with a relatively rigid structure due to
ring formation
Created with MindGenius Business 2005®
Advantages of fluorescence
Limit of detection is generally lower in
fluorescence than absorbance (103 time
lower)
Selectivity may also be better as not all
absorbing species fluoresce and there is
the choice of both excitation and
emission wavelength
Created with MindGenius Business 2005®
Created with MindGenius Business 2005®