Analysis of Glycosphingolipids
advertisement
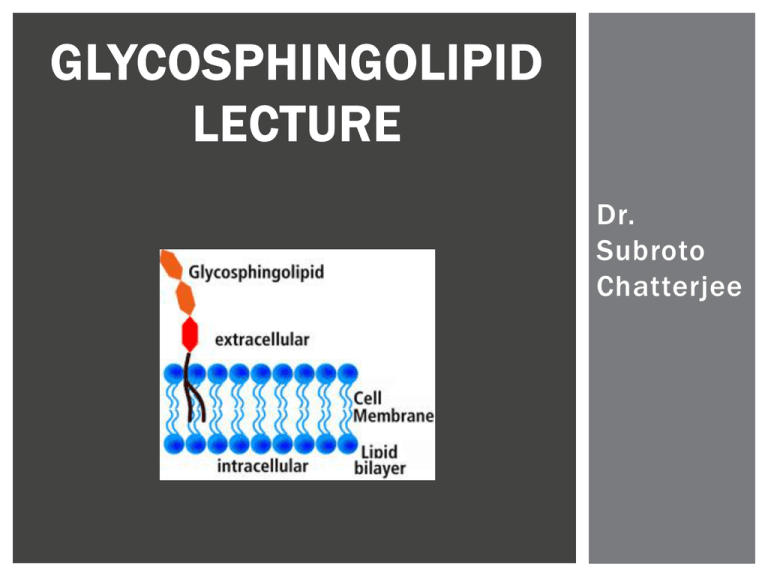
GLYCOSPHINGOLIPID LECTURE Dr. Subroto Chatterjee WHAT ARE GLYCOSPHINGOLIPIDS? The most common characteristic component of glycosphingolipids is the aliphatic amino alcohol discovered by Thudichum. Thudichum named it Sphingosine after the enigmatic Sphinx from Egypt having a head of Pharaoh and body of a lion. STRUCTURE OF LACTOSYLCERAMIDE STRUCTURE OF GLYCOSPHINGOLIPIDS LYSOSOMAL STORAGE DISORDERS Several metabolic basis of inherited diseases in man occur due to the lack/deficiency of enzymes which catabolize glycosphingolipids LYSOSOMAL STORAGE DISORDERS PREVALENCE OF GLYCOSPHINGOLIPIDS DISORDER Lysosomal Storage Disorders Prevalence Adrenoleukodystrophy (ADL) Niemann Pick (Type A,B, and C) Approx. 1 in 20,000 or 13,600 people in USA Gaucher Approx. 1 in 200 for general population High as 1 in 10 in Jewish people with East. European ancestry Krabbe Metachromatic leukodystrophy (MLD) Tay-Sachs Approx. 1 in 100,000 people Fabry Approx. 1 in 40,000 males Approx. 1 in 117,000 people for general population Type A & B: Approx. 1 in 250,000 Type C: Approx. 1 in 150,000 Approx. 1 in 625,000 people Approx. 1 in 27 Jewish people in USA Approx. 1 in 250 for general population GLYCOSPHINGOLIPID LECTURE TOPICS Function Methods for Determining Function Extraction and Purification Quantitation Structural Determination Localization Imaging Metabolism FUNCTION OF GLYCOSPHINGOLIPIDS 1) Superoxide generation and CAM expression 2) Inhibition of nitric oxide production in endothelial cells 3) As mediators of growth factors contributing to cell proliferation 4) As receptors for toxins and bacteria FUNCTION OF GLYCOSPHINGOLIPIDS IN ATHEROSCLEROSIS AND VASCULAR BIOLOGY 1) Generation of superoxide in arterial smooth muscle cells and expression of cell adhesion molecules. LACTOSYLCERAMIDE MEDIATES TNF ΑINDUCED ICAM-1 EXPRESSION IN ENDOTHELIAL CELLS LACTOSYLCERAMIDE STIMULATES SUPEROXIDE GENERATION IN HUMAN ENDOTHELIAL CELLS LACTOSYLCERAMIDE STIMULATES SUPEROXIDE GENERATION IN HUMAN ENDOTHELIAL CELLS FUNCTION OF GLYCOSPHINGOLIPIDS IN ATHEROSCLEROSIS AND VASCULAR BIOLOGY 2) Inhibition of nitric oxide production in endothelial cells. EFFECT OF LACCER ON ENDOTHELIUM DEPENDENT VASO-RELAXATION AND PORCINE CORONARY ARTERY EFFECTS OF LacCer ON eNOS mRNA LEVELS IN HCAECs. FUNCTION OF GLYCOSPHINGOLIPIDS IN ATHEROSCLEROSIS AND VASCULAR BIOLOGY 3. As mediators of growth factors contributing to cell proliferation and angiogenesis FUNCTION OF GLYCOSPHINGOLIPIDS 5) Serve as receptors to various toxins, e.g. cholera toxin and other bacteria METHODS FOR DETERMINING GSL FUNCTION Cellular assays Proliferation Adhesion Angiogenesis Migration Apoptosis METHODS FOR DETERMINING GSL FUNCTION METHODS FOR DETERMINING GSL FUNCTION GSL EXTRACTION 1) Bligh and Dyer 2) Folch Partitioning EXTRACTION OF GLYCOSPHINGOLIPIDS FROM HEART TISSUE • Modified Bligh & Dyer Method o https://encrypted-tbn0.gstatic.com/images?q=tbn:ANd9GcSoXJZC7o-xxP_UJqYeqM-GpLvFH_2Vq-18XdegMaMqdjDx9_V2 o o http://www.sfu.ca/bisc/bisc-429/folch.gif extraction in chloroform:methanol 2:1 homogenized by hand lipids extracted from organic phase Folch Partitioning PURIFICATION GLYCOSPHINGOLIPIDS Alkaline methanolysis The Glycolipid fraction from the silicic acid column is treated with mild base to remove contaminating phospholipids. This treatment does not affect glycolipids or gangliosides unless they contain an O-acyl group. The following quantities are used for 1-10mg of glycolipid fraction. Add 1ml of chloroform and 1ml of 0.6 N NaOH in methanol to the dry fraction and allow the mixture to react at room temperature for 1 hour. Then add 1.2 ml of 0.5 N HCL in methanol, 1.7ml of water, and 3.4ml of chloroform, mix well, centrifuge, and remove the lower layer containing the glycolipids. Was the lower layer three times with methanol:water (1:1) and then evaporate it to dryness in vacuo. HPTLC ANALYSIS OF NEUTRAL GSL A) Mixture of compounds in lane C through F B) Monohexosyl ceramide, gal- and glc-ceramide C) Dihexosyl ceramide, gal(1>4) glc-ceramide and gal(1->4)gal-ceramide D) Trihexosyl ceramide, gal(1>4)gal(1->4)glc-cermaide E) Tetrahexosyl ceramide galNAc(1->3)gal(1>4)gal(1->4)glc-ceramide F) On a silica gel H plate developed with chloroformmethanol-water (100:42:6) and visualized with alpha napthol spray HPTLC ANALYSIS OF GANGLIOSIDES Thin-layer chromatogram A) disaloganglioside, NANA (2>3)gal(1->3)galNAc(14) [NANA(2->3)]gal(1->4)glcceramide B) Monosialoganglioside, galNAc(1->4)[NANA(2->3)gal(1>4)glc-ceramide C) On a silica gel G (0.25mm) plate developed two times with chloroform -methanol-2.5N NH4OH (65:45:9) and visualized with alpha-naphtol spray QUANTITATION OF GLYCOSPHINGOLIPIDS Gas chromatography of sugars TMSI derivatization MS analysis TMSI derivatization HPLC analysis perbenzoylation (McCluer) endoglycoceramidation (Butters) HPTLC and densitometric scanning HPLC analysis deacylation (Zama) MS analysis Without derivatization QUANTITATION OF GLYCOSPHINGOLIPIDS Gas chromatography of sugars TMSI derivatization MS analysis TMSI derivatization HPLC analysis perbenzoylation (McCluer) endoglycoceramidation (Butters) HPTLC and densitometric scanning HPLC analysis deacylation (Zama) MS analysis Without derivatization TRIMETHYLSILYLATION AND GAS-LIQUID CHROMATOGRAPHY OF METHYL GLYCOSIDES QUANTITATION OF GLYCOSPHINGOLIPIDS QUANTITATION OF GLYCOSPHINGOLIPIDS QUANTITATION OF GLYCOSPHINGOLIPIDS QUANTITATION OF GLYCOSPHINGOLIPIDS QUANTITATION OF GLYCOSPHINGOLIPIDS QUANTITATION OF GLYCOSPHINGOLIPIDS Gas chromatography of sugars TMSI derivatization MS analysis TMSI derivatization HPLC analysis perbenzoylation (McCluer) endoglycoceramidation (Butters) HPTLC and densitometric scanning HPLC analysis deacylation (Zama) MS analysis Without derivatization MASS SPECTROMETRY OF INTACT TMSI DERIVATIVES OF GLYCOLIPIDS Mass spectrometr y of intact TMS derivatives of glycolipids gives information about the sugar groups, the fatty acid and the sphingosine por tion of glycosphingolipids. Bis (trimethylsiyl ) trifluroroacetamide (100microliter) and pyridine (50 microliter) are added to 20 -200microgram of the purified glycospingolipid in a small capped vial and heated at 60 degrees F for about 30 minutes. An aliquot containing 10 -20 microgram of the TMS glycolipid is evaporated to dr yness under nitrogen in a mass spectrometer direct probe tube. The samples are volatilized in the mass spectrometer ion source at temperatures ranging 100 degrees to 1 80 degrees depending on the size of the oligosacc haride unit. The following information can be obtained by comparison of the resulting mass spectra with those of reference samples: (1) whether the terminal residue is a hexose or hexosamine ; (2) the number of and nature of N acetylneuramine acid groups (i.e., terminal or branched); (3) whether N acetyl and/or N -glycol ylneuraminate is present; (4) information regarding the number of glycosyl residues present and the fatty acid and sphingosine composition. It is essential, because of the limitations of this technique (e.g., the inability to distinguish between hexoses), that it be used in conjuncti on with other techniques, such as permethylation analyses, and studies with specific glycosidases . QUANTITATION OF GLYCOSPHINGOLIPIDS Gas chromatography of sugars TMSI derivatization MS analysis TMSI derivatization HPLC analysis perbenzoylation (McCluer) endoglycoceramidation (Butters) HPTLC and densitometric scanning HPLC analysis deacylation (Zama) MS analysis Without derivatization PERBENZOYLATION Principle: Since neutral glycosphingolpids do not possess a characteristic chromophore that permits their quantitation by UV detection, they can be derivatized with benzoylchloride to form stable per -O,N benzoylated products. These products can be quantified by UV light at 280nm or at a higher sensitivity at 230nm. GSL(200ng) plus N -acetylpsychosine (an internal standard) are dried in N2. Perbenzoylation is carried out by adding 500uL of 10% benzoylchloride in pyridine for 16hr at 37 C. The samples are N2 dried and washed thrice with 1 .8mL of methanol: water(saturated with sodium carbonate). The hexane layer is washed and finally dried in N2. The derivatives are dissolved in CCL4(100uL) and a suitable aliquot injected in to the HPLC column(Zipax,E.I DuPont column 2.1 mmx500nm). McCluer: Methods in Enzymology 138: 1987 HPLC of male (C57BL/6J) mouse kidney perbenzoylated glycosphingolipids on a Zipax column with detection at 230nm QUANTITATION OF GLYCOSPHINGOLIPIDS Gas chromatography of sugars TMSI derivatization MS analysis TMSI derivatization HPLC analysis perbenzoylation (McCluer) endoglycoceramidation (Butters) HPTLC and densitometric scanning HPLC analysis deacylation (Zama) MS analysis Without derivatization QUANTITATION OF GLYCOSPHINGOLIPIDS Gas chromatography of sugars TMSI derivatization MS analysis TMSI derivatization HPLC analysis perbenzoylation (McCluer) endoglycoceramidation (Butters) HPTLC and densitometric scanning HPLC analysis deacylation (Zama) MS analysis Without derivatization QUANTITATION OF GLYCOSPHINGOLIPIDS 3) Endoglycoceramidase use to excise the oligosaccharides for quantification by HPLC. Wing, D. R., et al. "High-performance liquid chromatography analysis of ganglioside carbohydrates at the picomole level after ceramide glycanase digestion and fluorescent labeling with 2-aminobenzamide." Analytical biochemistry 298.2 (2001): 207-217. INCREASED GLYCOSPHINGOLIPID LEVELS IN SERUM AND AORTAE OF APOLIPOPROTEIN E GENE KNOCKOUT MICE QUANTITATION OF GLYCOSPHINGOLIPIDS Gas chromatography of sugars TMSI derivatization MS analysis TMSI derivatization HPLC analysis perbenzoylation (McCluer) endoglycoceramidation (Butters) HPTLC and densitometric scanning HPLC analysis deacylation (Zama) MS analysis Without derivatization QUANTITATION OF GLYCOSPHINGOLIPIDS Gas chromatography of sugars TMSI derivatization MS analysis TMSI derivatization HPLC analysis perbenzoylation (McCluer) endoglycoceramidation (Butters) HPTLC and densitometric scanning HPLC analysis deacylation (Zama) MS analysis Without derivatization QUANTITATION OF GLYCOSPHINGOLIPIDS USING DEACYLASE 2) deacylase treatment and quantification of lyso GSL Lipid Extraction Modified Bligh and Dyer N-deacylation Dried sphingolipid standards and samples were deacylated using sphingolipid ceramide N-deacylase (SCDase). To each dried sample and standard, 27uL 25mM sodium acetate buffer (pH 5.5) was added To this solution, 5uL SCDase was added Samples and standards were enzymatically digested for 19h The reaction was stopped with 200uL chloroform -methanol (1:1, v/v) The organic layer was removed and dried under N2 gas. Derivatization Samples were derivatized with 15uL OPA solution. HPLC Agilent 1260 Infinity using a Zorbax SB-C18 reversed-phase column Solvent System: isocratic (methanol:water acidified with 0.2% trifluoroacetic acid at 88:12, v/v) Flow Rate: 0.75 mL/min Ex λ: 340nm Em λ: 360nm QUANTITATION OF GLYCOSPHINGOLIPIDS Gas chromatography of sugars TMSI derivatization MS analysis TMSI derivatization HPLC analysis perbenzoylation (McCluer) endoglycoceramidation (Butters) HPTLC and densitometric scanning HPLC analysis deacylation (Zama) MS analysis Without derivatization QUANTITATION OF GLYCOSPHINGOLIPIDS Mass Spectrometry Without Derivatization Preparation Lipid extract spotted directly onto MALDI Opti-TOF plate with DHB matrix for positive/negative ion mode analysis GSL standards spotted Analysis Spectral ID for GSL ESTD precursor and product ions Comparison of ESTD to sample spectra Normalization to ISTD and protein concentration QUANTITATION OF GLYCOSPHINGOLIPIDS QUANTITATION OF GLYCOSPHINGOLIPIDS Normalize to concentration of ISTD QUANTITATION OF GLYCOSPHINGOLIPIDS Normalize to concentration of ISTD Calculate molar concentration (nmol/mg) using standard MW (968) and protein concentration from Bradford STRUCTURAL DETERMINATION A) Sequential digestion with exoglycosidases B) Deacylation to determine fatty acid composition C) Tandem mass spectrometry QTOF D) NMR STRUCTURAL DETERMINATION Sequential digestion with exoglycosidases STRUCTURAL DETERMINATION Deacylation to determine fatty acid composition - Dr. Chatterjee “TMSI Slide” STRUCTURAL DETERMINATION NMR - Allen Bush lecture LOCALIZATION OF GSL USING GALACTOSEOXIDASE Galactose oxdiase oxidizes D-galactosyl and N-acetylD-galactosaminyl residues at nonreducing terminals of glycoproteins and glycolipids to carbon -6 aldehydes. These aldehydes are then reduced back to galactose/N-acetylgalactosamine with tritiated borohydride. When intact cells are treated with the enzyme, only surface-exposed glycoconjugates are oxidized subsequently reduced, because the enzyme is unable to penetrate the cell plasma membrane. Because sialic acids often are linked to penultimate galactosyl residues, more efficient labeling is achieved by the simultaneous use of neuraminidase. LOCALIZATION OF GSL A) cell surface labeling using galactoseoxidase LOCALIZATION OF GSL B) Immunohistochemistry ELECTRON MICROSCOPY METABOLIC LABELING OF GSL use of radioactive serine, glucose, galactose , palmitate , acetate. Use of galactose oxidase to label cell surface GSL AUTORADIOGRAM OF GLYCOSPHINGOLIPIDS A : Gangliosides of mouse embryo secondary cells B : Neutral GSLs of mouse embryo secondary cells A. THIN-LAYER CHROMATOGRAM B.RADIOAUTOGRAM 1 ,5 – Standards of the neutral GSL fraction of human kidney were applied in channels 1 and 5. 2: 1-14C-N-acetylD-glucosamine 3: 1-14C-Dgalactose 4: 1-14C-Dglucosamine A. THIN-LAYER CHROMATOGRAM B. AUTORADIOGRAM 1,5 – Human brain gangliosides 2: 1-14C-Dglucosamine 3: 1-14C-Dgalactose 4: 1-14C-Dglucose GSL GLYCOSYLTRANSFERASE ASSAYS The Lactosylceramide Synthase Reaction GSL GLYCOSYLTRANSFERASE ASSAYS An essential feature of this galactosyltransferase is the requirement for manganese ions and detergent for optimal activity. This is because this is a Golgi –bound enzyme but is also present in the cell membrane. Radiolabeled UDP-galactose serves as the galactose donor and GlcCer as the acceptor. The pH optima is 7.8 and Tris or Cacodylate buf fer is preferred. Following incubation for 2hr at 37 C, the reaction is terminated with C:M 2:1 .The upper aqueous layer is discarded. The lower phase is N2 dried mixed with unlabeled LacCer and separated by HPTLC. The LacCer band id identified by exposing the HPTLC plate to iodine vapors. Gel area corresponding to LacCer is scraped and radioactivity s measured. The data is expressed as pmol LacCer synthesized /mg protein /2hr MEASUREMENT OF GLYCOSYLTRANSFERASE MASS Measurement of mass by: A) western immunoblot assay B) ELISA assay D - P DMP A LT ERS T H E E X P RESSION O F VA RI OUS G LYC OSYLTRA NSFE RASES A N D C OM P ONENT S O F S I G NA LI NG PAT HWAYS L E A DI NG TO C E LL P ROLI FERATI ON A N D A N G IOG ENE SIS I N A M O U SE M O D EL O F R E N A L C A N C ER . DELIVERY OF INHIBITORS OF GSL GLYCOSYLTRANSFERASE IN EXPERIMENTAL ANIMAL MODELS: Use of biopolymers, drug eluting stents DELIVERY OF INHIBITORS OF GSL GLYCOSYLTRANSFERASE IN EXPERIMENTAL ANIMAL MODELS: Use of a novel-antiproliferative compound coated on a biopolymer to mitigate platelet derived growth factor-induced proliferation in human aortic smooth muscle cells: comparison with sirolimus Yong-Dan Tang, Ambarish Pandey, Antonina Kolmakova, Xin-Tong Wang, Subbu S. Venkatraman, Subroto Chatterjee, Freddy Y.C. Boey: Glycoconjugate Journal 11/2008; 26(6):721-32. DELIVERY OF INHIBITORS OF GSL GLYCOSYLTRANSFERASE IN EXPERIMENTAL ANIMAL MODELS: 1 . Stent coating with PLGA and loading of DPDMP on the polymer Spraying fixture and enclosure Pre-spray materials preparations Metal stent preparation Airbrush or spray coating Stent sterilization and packaging for animal trial DELIVERY OF INHIBITORS OF GSL GLYCOSYLTRANSFERASE IN EXPERIMENTAL ANIMAL MODELS: 2. In vitro degradation and drug release Preparation of PLGA films and coated stents Drug quantification Degradation study REFERENCES Bligh, EG, and WJ Dyer. "Canadian Journal of Biochemistr y and Physiology." Canadian Journal of Biochemistr y and Physiology . 37.8 (1959): 911-917. Print. http://www.nrcresearchpress.com/doi/abs/10.1139/o59 -099 Chatterjee, S. Methods in Enzymology Chatterjee, S. and Mar tin, S Methods in Enzymology Wei, H and Chatterjee, S. Methods in Enzymology Mccluer , S. Methods in Enzymology Wing, D. R., et al. "High -per formance liquid chromatography analysis of ganglioside carbohydrates at the picomole level af ter ceramide glycanase digestion and fluorescent labeling with 2 aminobenzamide." Analytical biochemistr y 298.2 (2001): 207 -217. Zama, K, Y Hayashi, S Ito, Y Hirabayashi, T Inoue, K Ohno, N Okino, and M Ito. "Simultaneous quantification of glucosylceramide and galactosylceramide by normal-phase HPLC using O -phthalaldehyde derivatives prepared with sphingolipid ceramide N-deacylase." Glycobiology. 19.7 (2009): 767 -775. Print. doi: 10.1093/glycob/cwp047