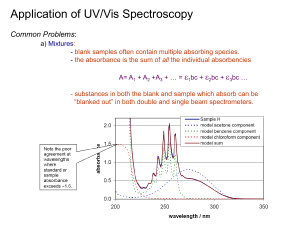
Determination of Concentration * Multicomponent System
advertisement

Applications of Spectrophotometry (Chapter 19) Red shift of lmax with increasing conjugation CH2=CHCH2CH2CH=CH2 lmax =185 nm CH2=CHCH=CH2 lmax =217 nm vs. Red shift of lmax with # of rings Benzene lmax =204 nm Naphthalene lmax =286 nm Polar solvents more likely to shift absorption maxima Shifts of lmax with solvent polarity n* hypsochromic/blue shift * bathochromic/red shift O Hypsochromic shift methanol heptane Generally, extending conjugation leads to red shift “particle in a box” QM theory; bigger box Substituents attached to a chromophore that cause a red shift are called “auxochromes” Strain has an effect… lmax 253 239 256 248 Generally, extending conjugation leads to red shift “particle in a box” QM theory; bigger box Absorbance Determination of Concentration – Multicomponent System C B l2 Wavelength (nm) Absorbance l1 C+B l1 l2 Wavelength (nm) Absorbance Determination of Concentration – Multicomponent System C B l1 l2 Wavelength (nm) (1) Measure eB and eC at l1 and l2 (pure substances) Determination of Concentration – Multicomponent System Absorbance (2) Measure A at l1 and l2 (mixture) C+B l1 l2 Wavelength (nm) Determination of Concentration – Multicomponent System A1 = xeB1 + yeC1 A2 = xeB2 + yeC2 Absorbance x = molarity B y = molarity C C+B l1 l2 Wavelength (nm) Isobestic Points HIn (acid form) In- (base form) Brocresol green When one absorbing species is converted to another, it is apparent in the absorption spectrum. Isobestic Points pH I see an isobestic point!!! pH [H+] [OH-] Isobestic Points In- (base form) HIn (acid form) The Total concentration of Bromocresol Green is constant throughout the reaction [Hin] + [In-] = F bromocresol green Cx + Cy = C A = b (exCx + eyCy) But at the isosbestic point both molar absorptivities are the same! e x + ey = e Isobestic Points In- (base form) HIn (acid form) ex + ey = e Therefore, the absorbance does not depend on the extent of reaction (i.e. on the particular concentrations of x and y) A = b (exCx + eyCy) = b e (Cx + Cy) = b e C An isobestic point is good evidence that only two principal species are present in a reaction. Isobestic Points - Application Oximetry 6.0E+05 5.0E+05 Hb deoxyhemoglobin HbO2 Oxyhemoglobin e (M-1cm-1) O2 4.0E+05 Hb02 3.0E+05 Hb 2.0E+05 1.0E+05 0.0E+00 250 450 650 850 l (nm) 2.0E+05 Hb02 1.8E+05 Hb 6.0E+03 1.6E+05 5.0E+03 1.2E+05 e (M-1cm-1) e (M-1cm-1) 1.4E+05 1.0E+05 8.0E+04 6.0E+04 Hb02 Hb 4.0E+03 3.0E+03 2.0E+03 100 % O2 4.0E+04 1.0E+03 2.0E+04 0.0E+00 400 600 800 l (nm) 1000 0 % O2 0.0E+00 600 700 800 l (nm) 900 1000 Measuring the equilibrium constant (The Scatchard Plot) Biochemistry example: The cellular action of a hormone begins when the hormone (L) Binds to it’s receptor protein (R) in a tight and specific way. The binding thus gives rise to conformational changes which change the biological activity of the receptor (an enzyme, an enzyme regulator, an ion channel, or a regulator of gene expression. R + L RL The binding depends of the concentration of the concentration of the components. Ka [ RL ] [ R ][ L ] kf kr 1 Kd Ka is the association constant and Kd is the dissociation constant Measuring the equilibrium constant (The Scatchard Plot) When binding has reached equilibrium, the total number of binding sites, Bmax = [R] + [RL] whereas, the number of unbound sites would be [R] = Bmax – [RL]. The equilibrium constant would then be Ka [ RL ] [ L ]( B max [ RL ]) This expression can then be rearranged to find the ratio of bound to unbound (free) hormone [ bound ] [ free ] [ RL ] [L] K a ( B max [ RL ]) 1 Kd ( B max [ RL ]) Measuring the equilibrium constant (The Scatchard Plot) [ bound ] [ free ] [ RL ] [L] K a ( B max [ RL ]) 1 Kd ( B max [ RL ]) The method of continuous variation (for isomolar solution) (Job’s method) A + nB ABn Absorbance Call this C Allows for the determination of the stoichiometry of the Predominant product. 0 Mole fraction (c = nb / na + nb) 1 The method of continuous variation (for isomolar solution) (Job’s method) Requirements: The system must conform to Beer's law. Only single equilibrium Equimolar solutions MA = MB Constant volume 1 = VT = VA + VB K reasonably greater than 1 pH and ionic strength must be maintained constant The method of continuous variation (for isomolar solution) (Job’s method) A Start Change Equil. + nB C M(1-x) Mx 0 -C -nC +C M(1-x)-C M(1-x) is a concentration Mx-nC M (1 x ) 1 C MV VT A Corrected Absorbance A3 X 0 0.25 AX 0.5 AX2 0.66 Mole fraction (c = nb / na) 1