Cell Transport Honors Biology Mr. Lee Room 320
Homeostasis, Osmosis,
Transport
Unit 6 – Chapter 5
Diffusion Through
Cell Boundaries
All living cells need a watery environment to survive!
The cell membrane helps organisms maintain Homeostasis
(Equilibrium) by controlling what substances enter or leave the cell
To remain alive, cells must maintain biological balance.
Cells maintain this balance
(homeostasis) in response to their immediate environment
Types of Cellular Transport
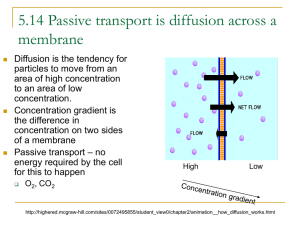
Passive Transport
CELL DOES NOT USE ENERGY
Diffusion
Osmosis
Facilitated Diffusion high
Active Transport
CELL DOES USE ENERGY
Protein Pumps
Endocytosis
Exocytosis high
Weeee!!
!
low low
This is gonna be hard work!!
3 Types of Passive
Transport
Diffusion – constant motion of molecules that causes them to spread out from high to low concentrations
Osmosis – diffusion of water
Facilitated Diffusion – diffusion with the help of transport proteins in the cell membrane
Diffusion
concentration
( concentration gradient)
Equilibrium occurs when the concentration of solute (particles) is the same throughout
(the particles still move!)
Because diffusion depends upon random particle movements
(kinetic energy) , substances diffusion
The Dye = Solute
Water= Solvent (In cells, water is always the Solvent).
Law of Diffusion
Substances ALWAYS diffuse from
HIGH to LOW concentrations.
This fact is key to understanding much of this chapter.
This is called moving DOWN the
Concentration Gradient.
OSMOSIS
Osmosis is the name for an important type of diffusion. It is the diffusion of water across the cell membrane. Since cells are usually bathed in a watery environment, they have to deal with water moving in/out of them. Too much water in or out of the cell can become a problem.
Osmosis – water moves from high to low concentration
100% pure water 90% water
10% salt level falls level rises
4 membrane
More water passes from
Pure water to salt solution...
...until water concentrations become equal
Water passes easily across membranes
Osmosis is the diffusion of water across a selectively permeable membrane
Osmosis exerts a pressure known as osmotic pressure on the hypertonic side of a selectively permeable membrane
Osmosis between cells
If the concentration of the cell sap is greater in one cell than in its neighbour, water will pass by osmosis from the less concentrated to the more concentrated.
cell sap more concentrated cell sap less concentrated
20
Osmosis in animal cells
There is a greater concentration of free water molecules outside the cell than inside so water diffuses into the cell by osmosis and the cell swells up
cell wall
Plant cells vacuole cytoplasm and cell membrane
The cell absorbs water by osmosis ....
....but the cell wall stops the cell expanding any more
Solutions
The relative concentrations of solutions to one another inside/outside of the cell can lead to 3 different situations. These situations are known as:
1.
Isotonic
2.
Hypertonic
3.
Hypotonic
** The next few slides will illustrate how these situations affect the cell.
Isotonic
Hypertonic
Solute concentration is greater outside the cell, so water moves OUT of the cell
Remember, hype
r
tonic, the cell sh r inks
The shrinking of cells is called Plasmolysis
Hypotonic
Solution concentration is greater inside the cell, so water moves
INTO the cell
Remember, hypotonic, the cell
POPS!!!
• The bursting of cells is called
Cytolysis
How Single Celled Critters
Deal with Osmosis
Unicellular organisms in hypotonic environments need to get rid of the excess water that diffuses into them
Contractile vacuoles are organelles that collect water and pump it out of the cell (uses energy)
How Multi-celled Critters
Deal with Osmosis
Other cells (especially in multicellular organisms) respond to a hypotonic environment by pumping solutes out of the cytoplasm
Water molecules are less likely to diffuse into the cell
Types of Passive Transport
(How cells transport materials in/out of themselves) – NO CELL ENERGY REQUIRED
1.
Osmosis
2.
Facilitated Diffusion
3.
Ion Channels
**Refer to the next 2 slides.
Facilitated Diffusion
Some molecules cannot diffuse through the cell membrane because they are:
Not soluble in lipids
Or are too large to pass through the pores in the membrane (I.E.
Glucose)
These molecules are helped across the membrane by carrier proteins
The carrier proteins change shape after the molecule binds
Diffusion Through
Ion Channels
Ions such as sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl-) are important for cell functions
Since they are not soluble in lipids they will not pass through the cell membrane on their own
Diffusion Through
Ion Channels…
Ion channels provide small tunnels across the cell membrane
Each type of ion channel is usually specific for one type of ion
Some channels are always open, some are gated
The gates respond to three stimuli:
Stretching of the cell membrane
Electrical signals
Chemicals in the cytosol or external environment
No energy is used, so it is still passive
Active Transport – (cells actively work to move some substances in/out) – CELL
ENERGY IS REQUIRED
1.
Pumps in the cell membrane – proteins in the cell membrane use cell energy to change their shape to actively pump molecules in/out of cell. Ex.)
Sodium/Potassium Pump.
2.
Endocytosis – moving very large molecules INTO the cell. Cell wraps its membrane around the large molecule. This requires the cell to spend energy.
3.
Exocytosis – moving large OUT OF the cell. Cell membrane changes its shape to push molecule out of cell. This requires cell energy .
***See pages 101 to 104 in book.