Unit 10 - Thermochemistry
advertisement

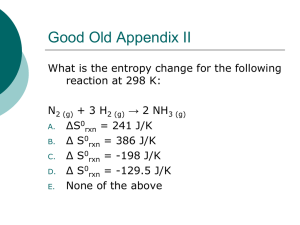
Thermodynamics & Thermochemistry Unit 10 Overview • Energy – – – – – – Units Heat /temperature System/surrounding Calorimeters 1st Law Thermodynamics Heat Capacity • Enthalpy – – – – – – ∆H and ∆H° Endothermic/exothermic Heat of formation Stoichiometry Bond energy Hess’s Law • Entropy – – – – ∆S and ∆S° Spontaneous reactions Entropy for phase changes 2nd Law of Thermodynamics • Gibbs Free Energy – ∆G and ∆G° – ∆G = ∆H - T∆S – Spontaneous reactions • State functions What is Thermodynamics? The study of the energy and its transformations What is Thermochemistry? The study of the transfers of energy as heat that accompany chemical reactions and physical changes Energy • The capacity to do work or to transfer heat – Work: the energy used to cause an object with mass to move against a force – Heat: the energy used to cause the temperature change in an object • Units of Energy – Joule (J) = 1 kg∙m2/s2 – Calorie (cal) = 4.184 J • In nutrition 1 calorie (Cal) = 1000 cal • (notice difference is that nutrition calorie is capitalized) • In chemistry 1000 cal = 1 kcal What is the difference between heat and temperature? What’s the difference? HEAT • Energy transferred between samples of matter because of a difference in their temperatures • Cannot be measured directly (determined by temperature change) • Joules (J) = 1 kg∙m2/s2 • Moves from high to low temperature TEMPERATURE • Measure of the average kinetic energy of the particles in a sample of matter • Directly measureable (affected by heat transfer) • Celsius (°C) or Kelvin (K) • Higher kinetic energy = higher temperature How do we measure energy? Calorimeter Measures energy absorbed or released as heat in a chemical/physical change Constant Pressure Example “coffee-cup” calorimeter uses unsealed calorimeter where reaction occurs under atmospheric pressure How do we measure energy? Constant Volume Example “bomb” calorimeter uses pressurized sealed vessel called “bomb” submerged in water System and Surroundings • System: portion we single out for studying – Open system: matter and energy exchanged with surroundings – Closed system: energy exchanged within system • Surroundings: everything else that is not included in the system Work E = q + w E = change in internal energy of a system q = heat flowing into or out of the system -q if energy is leaving the system +q if energy is entering the system w = work done by, or on, the system -w if work is done by the system on the surroundings +w if work is done on the system by the surroundings Pressure-Volume Work • Most common type of work. – Example: A sample of gas is held by a pressure of 2.4 atm. The pressure is then changed to 1.3 atm. The gas expands. How much work is done? – Note that because the gas is expanding, the system is doing work on the surroundings so work must be negative Pressure-Volume Work When the volume (V) of a system increases by an amount of ΔV against an external pressure (p), the system pushes back, and thus does pV work on the surroundings w = -PΔV Pressure-Volume Work w PV Expansion Compression +V (increase) -V (decrease) -w results E system decreases Work has been done by the system on the surroundings +w results E system increases Work has been done on the system by the surroundings FIRST LAW OF THERMODYNAMICS Energy is neither created nor destroyed (conservation of energy) FIRST LAW OF THERMODYNAMICS • Energy is transferred but not lost • Energy lost by a system must be gained by surroundings and vice versa What determines how much heat is transferred during a temperature change? Amount depends on… 1. Nature of material changing temperature. 2. Mass of material changing temperature. 3. Size of the temperature change. Example 1 Example 2 One gram of each of two metals (iron and silver) are heated to 100°C and cooled to 50°C in a beaker of water. Iron transfers 22.5 J of energy. Silver transfers 11.8 J of energy. What makes the difference? Heat Capacity • Heat Capacity (Cp) – temperature change experienced by an object when it absorbs a certain amount of heat – The greater the heat capacity, the greater the heat required to produce given increase in temperature Heat Capacity Cp = ∆H ∆T Cp = heat capacity ∆H = heat added (J or cal) ∆T = temperature change (°C or K) Heat Capacity (Pure Substances) • Molar Heat Capacity (Cm): Amount of energy required to raise the temperature of 1 mole of a substance by 1 degrees – Celsius or Kelvin • Specific Heat (Cs): Amount of energy required to raise the temperature of 1 gram of a substance by 1 degrees – Celsius or Kelvin • Higher specific heat means… – Higher heat capacity – Heats up slower – Transfers more heat Specific Heat Explain in terms of specific heat Calculations q = mc∆T q = heat added (J or cal) m = mass of substance (g or kg) c = specific heat ∆T = temperature change (°C or K) Specific Heat (Units) J/g°C or J/gK Example 1 A 4.0 g sample of glass was heated from 274 K to 314 K and was found to have absorbed 32 J of heat. A. What is the specific heat of this type of glass? B. How much energy will the same glass sample gain when it is heated from 314 K to 344 K? Example 2 A piece of copper alloy with a mass of 85.0 g is heated from 30.°C to 45°C. In the process, it absorbs 523 J of energy as heat. A. What is the specific heat of this copper alloy? B. How much energy will the same sample lost if it is cooled from 45°C to 25°C? Enthalpy What is Enthalpy? The amount of energy absorbed by a system as heat during a process at constant pressure. How does it apply to reactions? Enthalpy Change Difference between enthalpies of products and reactants H = Hproducts - Hreactants Enthalpy of Reaction Quantity of energy transferred as heat during chemical reaction (“heat of reaction”) What’s the difference? Endothermic Exothermic • Heat absorbed • Heat released • Energy gained • Energy lost When you gamble and lose your money, do you end up with less money or more? ….do you have a “positive or negative” amount of money in your pocket after? When you gamble and win money, do you end up with less money or more? ….do you have a “positive or negative” amount of money in your pocket after? What’s the difference? Endothermic Exothermic • Heat absorbed • Heat released • Energy gained • Energy lost • H = positive (+) • H = negative (-) • H2O + 483.6 kJ H2 + O2 • H2O H2 + O2 H = +483.6 kJ • H2 + O2 H2O + 483.6 kJ • H2 + O2 H2O H = -483.6 kJ Energy Diagrams • Activation Energy – energy needed for reactants to transition to products (become activated complex) • At activated complex, all bonds have been broken but no bonds formed (highest energy/lowest stability) • Exothermic diagram energy of products are lower than energy of reactants (∆H is negative) Energy Diagrams • Activation Energy – energy needed for reactants to transition to products (become activated complex) • At activated complex, all bonds have been broken but no bonds formed (highest energy/lowest stability) • Endothermic diagram energy of products are higher than energy of reactants (∆H is positive) Most reactions in nature are exothermic… Hf = negative When Hf positive or slightly positive, the compound is unstable… …very positive Hf can even be explosive For example: Mercury fulminate is used as a detonator in explosives. HgC2N2O2 Hf = + 270 kJ/mol Standard State Conditions • • • • • All gases are at 1 atm All liquids are pure All solids are pure All solutions are at 1 M concentration Temperature is room temperature (25°C or 298K) unless told otherwise • Energy of formation of element in its normal state = 0 • Standard state conditions indicated by superscript 0 – Example: ∆H° means standard state conditions Formation of Compounds Enthalpy of Formation (Hf) – Heat of Formation Enthalpy change that occurs when 1 mole of a compound is formed from its elements (sometimes called molar enthalpy of formation) To represent standard state use Hf0 Elements in their standard state have Hf0 = 0 Heat of Formation • To form compounds from elements or compounds in their standard states break apart compounds into individual components – Balance by using fractions on standard state elements/compounds rather than whole numbers Example 1: Equation for formation of NaCl(s) Na(s) + ½ Cl2(g) NaCl(s) Example 2: Equation for formation of CaCO3(s) Ca(s) + C(s) + 3/2 O2(g) CaCO3(s) Heat of Formation Hf0 = ∑ Hf0 products - ∑ Hf0 reactants Example: 2CH3OH(g) + 3O2(g) 2CO2(g) + 4H2O(g) Hf0 = [2(-394) + 4(-242)] – [2(-201) + 3(0)] Hf0 = [-1756] – [-402] Compound Hf0 = -1354 kJ CH3OH(g) ∆H (kJ/mol) -201 O2(g) 0 CO2(g) -394 H2O(g) -242 Enthalpy Stoichiometry How much heat is released when 5.40 g of methane gas is burned in a constant-pressure system? CH4(g) + 2O2(g) CO2(g) + 2H2O(l) ∆H = -890 kJ 4.50 g CH4 × 1 mol CH4 × -890 kJ = - 250 kJ 16.0 g CH4 1 mol CH4 Bond Energy • Energy required to break a bond. – Breaking a bond requires energy (endothermic) • Bond energy is positive – Forming a bond releases energy (exothermic) • Bond energy is negative H0 = ∑ Bond Energies of bonds broken – ∑Bond Energies of bonds formed H0 = ∑ Bond Energyreactants – ∑ Bond Energyproducts Bond Energy H0 = ∑ Bond Energies of bonds broken – ∑Bond Energies of bonds formed Example: 2H2(g) + O2(g) 2H2O(g) H0 = [2(H-H) + 1(O-O)] – [4(O-H)] H0 = [2(436) + 1(499)] – [4(463)] H0 = -481 kJ/mol Bond Bond Energy (kJ/mol) H-H 436 O=O 499 O-H 463 Sometimes finding the enthalpy change in reactions is difficult or even impossible due to the reaction. How can we find Hf for these types of reactions? Hess’s Law The overall enthalpy change in a reaction equals the sum of the enthalpy changes for individual steps in the process. Can be used to find the Hf for reactions that are difficult or impossible to measure. Example C(s) + 2H2(g) CH4(g) Hf0 = ? We can use the following to help… C(s) + O2(g) CO2(g) H2(g) + ½O2(g) H2O(l) CH4(g) + 2O2(g) CO2(g) + 2H2O(l) Hf0 = -393.5 kJ Hf0 = -285.8 kJ Hf0 = -890.8 kJ Example Rules to remember: 1. When a reaction is reversed, the sign of Hf must be reversed (+/-). 2. When coefficients are multiplied by a number, Hf must be multiplied by the same number. Example C(s) + O2(g) CO2(g) H2(g) + ½O2(g) H2O(l) CH4(g) + 2O2(g) CO2(g) + 2H2O(l) C(s) + 2H2(g) CH4(g) Hf0 = -393.5 kJ Hf0 = -285.8 kJ Hf0 = -890.8 kJ Hf0 = ? Example C(s) + O2(g) CO2(g) 2H2(g) + O2(g) 2H2O(l) CH4(g) + 2O2(g) CO2(g) + 2H2O(l) C(s) + 2H2(g) CH4(g) Hf0 = -393.5 kJ Hf0 = 2(-285.8 kJ) Hf0 = -890.8 kJ Hf0 = ? Multiply reaction and Hf0 by 2. Example C(s) + O2(g) CO2(g) 2H2(g) + O2(g) 2H2O(l) CO2(g) + 2H2O(l) CH4(g) + 2O2(g) C(s) + 2H2(g) CH4(g) Hf0 = -393.5 kJ Hf0 = 2(-285.8 kJ) Hf0 = +890.8 kJ Hf0 = ? Reverse reaction and change the sign of Hf0 Example C(s) + O2(g) CO2(g) 2H2(g) + O2(g) 2H2O(l) CO2(g) + 2H2O(l) CH4(g) + 2O2(g) C(s) + 2H2(g) CH4(g) Hf0 = -393.5 kJ Hf0 = 2(-285.8 kJ) Hf0 = +890.8 kJ Hf0 = -74.3 kJ Add the Hf0 of each reaction in the process to determine the final Hf0 . Elements that appear on both sides of the reaction cancel out. Practice 2N2(g) + 5O2(g) 2N2O5(g) Hf0 = ? Determine the Hf0 for the above reaction using the following process… H2(g) + ½O2(g) H2O (l) N2O5 (g) + H2O (l) 2HNO3(l) ½N2(g) + O2(g) + ½H2(g) HNO3(l) Hf0 = -285.8 kJ Hf0 = -76.6 kJ Hf0 = -174.1 kJ What predicts whether a reaction will occur spontaneously? 1. Change in energy (H) Most reactions in nature are exothermic… The goal is to end up in a lower energy state (products having lower energy than reactants) Can spontaneous reactions be endothermic? Think about ice… Energy is transferred from the warm air to the ice The well ordered ice cube crystal melts to the less ordered liquid phase having a higher energy What is Entropy? Measure of the degree of randomness of particles in a system (S) Increase in entropy usually means increase in… • Temperature • Volume • Number of independently moving particles The more random the system, the higher the entropy. Low Entropy Higher Entropy Highest Entropy •Particles in solution have higher entropy values than particles in solids •Two moles of a substance have a higher entropy value than 1 mole Entropy (S) for phase changes ∆S = ∆H T • ∆S = entropy of fusion or vaporization (J/K) • ∆H = heat of vaporization or fusion (J/mol) • T = Kelvin temperature (Multiply ∆H by moles to get units of J) Entropy (S) for phase changes Example: The normal freezing temperature for liquid mercury is -38.9°C and its molar enthalpy of fusion, ∆Hfusion, is 2.29kJ/mol. What is the entropy change of the system when 50.0 g of liquid mercury freezes at the normal freezing point? 50.0 g Hg = 0.25 moles Hg ∆H ∆S = T -2.29 kJ/mol = -2290 J/mol (0.25 mole)(-2290 J/mol) = = - 2.44 J/K 234.3 K Change in Entropy (S) The difference between the entropy of the products and reactants S0 = ∑ S0 products - ∑ S0 reactants Increase in entropy S is positive (+) Decrease in entropy S is negative (-) Units: kJ/molK Standard Molar Entropy (S0) • S0 of elements in their normal state at 298K are not zero (unlike H0) • Gases > liquids > solids • Increase with increasing molar mass • Increase with increasing number of atoms in the formula of a substance SECOND LAW OF THERMODYNAMICS The entropy of the universe always increases during spontaneous reactions (If a reaction is spontaneous in one direction it cannot be spontaneous in the reverse direction) SECOND LAW OF THERMODYNAMICS ∆Suniverse = ∆Ssystem + ∆Ssurroundings • Any irreversible process results in an increase in entropy (∆S > 0) • Any reversible process results in no overall change in entropy (∆S = 0) What predicts whether a reaction will occur spontaneously? 1. Change in energy (H) 2. Randomness of particles (S) Which is preferred? LEAST Enthalpy (H) _______ MOST Entropy (S) _______ The interaction between the two determines the change in a system (spontaneous or not) Gibbs Free Energy (G) The combined enthalpy-entropy function in a system Natural processes have a lower free energy (negative) Gibbs Free Energy G0 = ∑ Gf0 products - ∑ Gf0 reactants ∆ G, ∆ H, and ∆S Difference between change in enthalpy (H) and the product of the Kelvin temperature and the entropy change (TS) G = H - TS T = Kelvin temperature What makes a reaction spontaneous? If G < 0 the reaction is spontaneous H S G – – + + – + – – (low temp) – (high temp) + – + If G = 0 then the reaction is at equilibrium. Example For the following reaction NH4Cl(s) NH3(g) + HCl(g) At 298.15 K, H0 = 176 kJ/mol and S0 = 0.285 kJ/molK. Calculate G0 and tell whether the reaction is spontaneous. State Functions • Property of a system determined by the system’s condition (state) in terms of pressure, temperature, etc. – Depends only on present state of system, not the path it took to get there – Enthalpy, entropy, Gibbs Free Energy are state functions • Example: When traveling to Denver from Chicago, Denver is 5280 ft above sea level and Chicago is 596 ft above sea level. No matter what route you take, the altitude change will be 4684 ft. Altitude is a state function. (distance is not)