Chapter 15 Chemical thermodynamic
advertisement

Chapter 15
Chemical
Thermodynamics
化學熱力學
1
Outline
Heat Changes and Thermochemistry
1.
2.
3.
4.
5.
6.
7.
The First Law of Thermodynamics 熱力學第一定律
Some Thermodynamic Terms
Enthalpy Changes 焓的變化
Calorimetry 熱量的測量
Thermochemical Equations 熱化學方程式
Standard States and Standard Enthalpy Changes
Standard Molar Enthalpies of Formation, Hfo
8. Hess’s Law
9. Bond Energies
10.Changes in Internal Energy, E
11.Relationship of H and E
2
Outline
Spontaneity of Physical and Chemical Changes
12. The Two Aspects of Spontaneity
13. Dispersal of Energy and Matter
14. Entropy熵, S, and Entropy Change, S
15. The Second Law of Thermodynamics
16. Free Energy Change, G, and Spontaneity
17. The Temperature Dependence of Spontaneity
3
The First Law of Thermodynamics
熱力學第一定律
• Thermodynamics is the study of the changes in
energy and transfers of energy that accompany
chemical and physical processes. 所謂的熱力學是
指在化學及物理反應中能量的改變及轉移
• In this chapter we will address 3 fundamental
questions.
1.Will two (or more) substances react when they
are mixed under specified conditions?當兩個物質在
特定狀態混合時,是否會發生反應?
2.If they do react, what energy changes and
transfers are associated with their reaction?如果發
生反應,那反應中有那些能量的改變及轉移?
3.If a reaction occurs, to what extent does it occur?
4
The First Law of Thermodynamics
Energy is the capacity to do work or to transfer heat.
•Exothermic reactions release energy in the form of
heat. (放熱反應以熱的形式釋出能量)
–For example, the combustion of propane is
exothermic. (丙烷的燃燒為放熱反應)
•C3H8(g) + 5 O2(g) 3 CO2(g) + 4 H2O(l) + 2.22x103 kJ
–The combustion of n-butane is also exothermic (正丁
烷的燃燒亦為放熱反應)
•2 C4H10(g) + 13 O2(g) 8 CO2(g) + 10 H2O(l) + 5.78x103 kJ
烷類和過量的氧作用會形成二氧化碳和水
• A process that absorbs energy from its surroundings is
called endothermic.
– H2O(s) + 6.02kJ H2O(l)
5
The First Law of Thermodynamics
•Exothermic reactions
generate specific amounts
of heat.(放熱反應產生熱)
活化能
(activation energy)
– This is because the potential
energies of the products are
lower than the potential
energies of the reactants. (
此乃因產物的位能比反應物未
能來得低)
Kinetic energy 動能
v.s.
Potential energy 位能
(包含化學能)
6
The First Law of Thermodynamics
• There are two basic ideas of importance for
thermodynamic systems. (熱力學系統有兩個最基本的概
念)
1. Chemical systems tend toward a state of
minimum potential energy.(系統傾向最小位能)
Some examples of this include:
H2O flows downhill.
Objects fall when dropped.
The energy change for these two examples is:
Epotential = mgh
Epotential = mg(h)
7
The First Law of Thermodynamics
2. Chemical systems tend toward a state of
maximum disorder. (系統傾向最大亂度)
• Common examples of this are:
– A mirror shatters when dropped and does not reform.
– It is easy to scramble an egg and difficult to
unscramble it.
– Food dye when dropped into water disperses.
8
The First Law of Thermodynamics
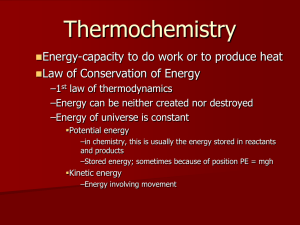
• This law can be stated as, “The combined amount
of matter and energy in the universe is constant.” (熱
力學第一定律可定義為物質和能量的總和不會改變)
• The first law is also known as the Law of
Conservation of Energy (即為能量守恆定律).
– Energy is neither created nor destroyed in chemical
reactions and physical changes. 在化學及物理反應時,能
量既不會憑空消失,也不會憑空產生,只能從一種形式轉化成另
一種形式,或者從一個物體轉移到另一個物體,而總量保持不變
9
Some Thermodynamic Terms
•The substances involved in the chemical and physical
changes under investigation are called the system.
– In chemistry lab, the system is the chemicals inside the beaker.
•The environment around the system is called the
surroundings.
– The surroundings are outside the beaker.
•The system plus the surroundings is called the universe.
•The set of conditions that specify all of the properties of the
system is called the thermodynamic state of a system.
•For example the thermodynamic state could include:
–
–
–
–
The number of moles and identity of each substance.
The physical states of each substance.
The temperature of the system.
The pressure of the system.
10
Some Thermodynamic Terms
•The properties of a system that depend only on the state
of the system are called state functions.(狀態函數指一個系統的
特質只與狀態有關而與途徑無關, 通常以大寫表示)
– The value of a state function is independent of pathway.
– An analog to a state function is the energy required to climb
a mountain taking two different paths. (走兩條不同的路而到達山
頂的位能差相同,但路徑不同)
• E1 = energy at the bottom of the mountain E1 = mgh1
• E2 = energy at the top of the mountain
E2 = mgh2
• E = E2-E1 = mgh2 – mgh1 = mg(h)
– Notice that the energy change in moving from the top to the
bottom is independent of pathway but the work required
may not be!
– Some examples of state functions are:
•T, P, V, E, H, and S
– Examples of non-state functions are:
•n, q, w
11
Some Thermodynamic Terms
• In
thermodynamics we are often interested in
changes in functions.
– We will define the change of any function X as:
– X = Xfinal – Xinitial
• If X increases X > 0
• If X decreases X < 0.
12
Enthalpy Change 焓變化 (反應熱)
•Chemistry is commonly done in open beakers on a
desk top at atmospheric pressure.
–Because atmospheric pressure only changes by
small increments, this is almost at constant
pressure.
•The enthalpy change, H, is the change in heat
content at constant pressure.焓變化是指定壓下熱含量
的變化
–H = qP
13
Enthalpy Change
• Hrxn is the heat of reaction. 反應熱
– This quantity will tell us if the reaction produces or
consumes heat. (Hrxn的量表示反應產生熱或消耗熱)
– If Hrxn < 0 the reaction is exothermic.
– If Hrxn > 0 the reaction is endothermic.
• Hrxn = Hproducts - Hreactants
– Hrxn = Hsubstances produced - Hsubstances consumed
– Notice that this is Hrxn = Hfinal – Hinitial
14
Calorimetry 熱量的測量
• A coffee-cup calorimeter (
熱卡計) is used to measure
the amount of heat
produced (or absorbed) in
a reaction at constant P
– This is one method to
measure qP for reactions in
solution.
15
Calorimetry
• If an exothermic reaction is performed in a
calorimeter, the heat evolved by the
reaction is determined from the temperature
rise of the solution.
– This requires a two part calculation.
Amount of heat
Amount of heat
Amount of heat
+
Released by reaction = absorbed by calorimeter
absorbed by solution
• Amount of heat gained by calorimeter is called the
heat capacity of the calorimeter or calorimeter
constant.
– The value of the calorimeter constant is determined by
adding a specific amount of heat to calorimeter and
measuring the temperature rise.
Heat absorbed (J)
Heat capacity (C) =
Increase in temperature (oC)
所謂熱容量(heat capacity)是指使一個物體上升1oC所需的熱量
16
Calorimetry
Example 15-1: When 3.425 kJ of heat is added to a
calorimeter containing 50.00 g of water the temperature rises
from 24.00oC to 36.54oC. Calculate the heat capacity of the
calorimeter in J/oC. The specific heat of water is 4.184 J/g oC.
水的溫度變化 T = (36.54 – 24.00)oC = 12.54oC
水所吸收的熱 qp = mCT
= (50.00g) (4.184 J/goC) (12.54oC)
= 2623.37 J
熱卡計所吸收的熱量 = 3425J – 2623J
= 802 J
熱卡計的熱容量 = 802J / 12.54oC
= 64.0 J/oC
17
Example 15-2: A coffee-cup calorimeter is used to determine the
heat of reaction for the acid-base neutralization
CH3COOH(aq) + NaOH(aq) NaCH3COO(aq) + H2O()
When we add 25.00 mL of 0.500 M NaOH at 23.000oC to 25.00 mL of
0.600 M CH3COOH already in the calorimeter at the same
temperature, the resulting temperature is observed to be 25.947oC.
(The heat capacity of the calorimeter : 27.8 J/0C. The specific heat
of the mixture is 4.18 J/g0C and the density: 1.02 g/mL.)
溫度變化 T= 25.947 – 23.000 = 2.947 oC
熱卡計所吸收的熱量 q= (2.947oC)(27.8 J/oC) = 81.9 J
混合溶液的重量 = (25.00ml + 25.00ml) x1.02 g/ml = 51.0g
溶液所吸收的熱量 q= mCT
= (51.0g)(4.18 J/goC)(2.947oC)
= 628 J
反應所產生的總熱量為 = 81.9J + 628J = 709.9J
CH3COOH(aq) + NaOH(aq) NaCH3COO(aq) + H2O()
1
1
1
25.00mlx0.5 M 25.00mlx0.6 M
12.5mmol
=12.5mmol
=15.0mmol
12.5mmol = 0.0125 mol
指每莫耳所放出的熱量
Hrxn = 709.9J / 0.0125 mol
= 56792 J/mol = 5.68kJ/mol
18
Thermochemical Equations
熱化學方程式
•Thermochemical equations are a balanced
chemical reaction plus the H value for the reaction.
(熱化學方程式為平衡的化學式再加上反應所需的熱量H.)
– For example, this is a thermochemical equation.
C5H12(g) + 8 O2(g) 5 CO2(g) + 6 H2O(l) + 3523 kJ
1mole 8moles
5moles 6moles
•1 mol of C5H12 reacts with 8 mol of O2 to produce 5 mol of
CO2, 6 mol of H2O, and releasing 3523 kJ is referred to as
one mole of reactions.
•This is an equivalent method of writing thermochemical
equations.
C5H12(g) + 8 O2(g) 5 CO2(g) + 6 H2O(l) Horxn= - 3523 kJ
– H < 0 designates an exothermic reaction.
– H > 0 designates an endothermic reaction
19
Thermochemical Equations
Example 15-3: When 2.61g of dimethyl ether (CH3OCH3) is
burned at constant pressure, 82.5 kJ of heat is given off.
Find H for the reaction.
CH3OCH3(l) + 8 O2(g) 2 CO2(g) + 3 H2O(l)
CH3OCH3 的分子量為 46
CH3OCH3 的 mole數 = 2.61g/ 46
= 5.67x10-2 mole
此反應為放熱反應
Hrxn = - (82.5 kJ/ 5.67x10-2 mol)
= -1455 kJ/ mol rxn
20
Thermochemical Equations
Example 15-4: When aluminum metal is exposed to
atmospheric oxygen, it is oxidized to form aluminum
oxide. How much heat is released by the complete
oxidation of 24.2 g of aluminum at 25oC and 1atm?
4 Al(s) + 3 O2(g) 2 Al2O3(s) H = -3352 kJ/mol rxn
Al 的原子量為 27
Al 的 mole數 = 24.2g/ 27
= 0.896 mole
Hrxn = - 3352 kJ/mol rxn
表示1mole的反應所釋出的熱量
而1mole的反應需4mole的Al
0.896 mol
x kJ
=
4 mol Al
-3352 kJ
x= -751kJ
21
Standard States and Standard Enthalpy
Changes 標準狀態及標準焓變
•Thermochemical standard state conditions
–The thermochemical standard T = 298.15 K. (25oC)
–The thermochemical standard P = 1.00 atm.
•例如標準狀態下(1大氣壓, 25oC),H2(g), Hg(l), Na(s),
C2H5OH(l), CaCO3(s), CO2(g)
•Thermochemical standard states of matter
– For pure substances in their liquid or solid phase the
standard state is the pure liquid or solid.
– For gases the standard state is the gas at 1.00 atm of
pressure.
• For gaseous mixtures the partial pressure must be 1.00 atm.
– For aqueous solutions the standard state is 1.00 M
concentration.
• Standard enthalpy change, Horxn All at standard 22
states (只看反應前、反應後,不管反應過程狀態是否改變)
Standard Molar Enthalpies of Formation,
標準莫耳生成焓,Hfo
•The standard molar enthalpy of formation is defined
as the enthalpy for the reaction in which one mole of
a substance is formed from its constituent elements. (
在標準狀態下由各元素形成一莫耳物質所需的熱量及稱之)
– The symbol for standard molar enthalpy of formation (
簡稱heat of formation) is Hfo.
•The standard molar enthalpy of formation for MgCl2 is:
Mg(s) + Cl2(g) MgCl2(s) + 64.18 kJ
HofMgCl2 = -641.8 kJ/mol MgCl2(s)
•The standard molar enthalpy of formation for HBr is:
H2(g) + Br2(l) 2HBr(g) Horxn = -72.8 kJ/mol rxn
½ H2(g) + ½ Br2(l) HBr(g) Horxn = -36.4 kJ/mol
HofHBr = -36.4 kJ/mol HBr(g)
23
24
Standard Molar Enthalpies of
Formation 標準莫耳生成焓, Hfo
• Standard molar enthalpies of formation have been
determined for many substances and are tabulated
in Table 15-1 (Table6.2) and Appendix 4 in the text.
• Standard molar enthalpies of elements in their most
stable forms at 298.15 K and 1.000 atm are zero.
Example 15-5: The standard molar enthalpy of
formation for phosphoric acid is -1281 kJ/mol. Write the
equation for the reaction for which Horxn = -1281 kJ.
P in standard state is P4
Phosphoric acid in standard state is H3PO4(s)
3/2 H2(g) + 2 O2(g) + 1/4 P4(s) H3PO4(s) + 1281 kJ
HofH3PO4 = -1281 kJ/mol
25
Standard Molar Enthalpies of
Formation, Hfo
Example 15-6: Calculate the enthalpy change for the
reaction of one mole of H2(g) with one mole of F2(g)
to form two moles of HF(g) at 25oC and one
atmosphere.
H2(g) + F2(g) 2HF(g)
Std. state
Std. state
Std. state
for this rxn HoHF = 2 Hof
查表得知: Hof = -271 kJ/mol
所以 HoHF = (2mol)(-271kJ/mol)
= -542 kJ
26
Standard Molar Enthalpies of
Formation, Hfo
• Example 15-6: Calculate the enthalpy change for
the reaction in which 15.0 g of aluminum reacts with
oxygen to form Al2O3 at 25oC and one atmosphere.
HofAl2O3 = -1676 kJ/mol Al2O3
2 Al(s) + 3/2 O2(g) Al2O3(s)
Al mole = 15.0g/ 27
= 0.556 mole
0.556 mol
x kJ
=
2 mol Al
-1676 kJ
x= -466kJ
27
Hess’s Law
• Hess’s Law of Heat Summation states that the
enthalpy change for a reaction is the same
whether it occurs by one step or by any
(hypothetical) series of steps.
– Hess’s Law is true because H is a state function.
赫士定律(Hess’s law,又名反應熱加成性定律): 若一反應為
二個反應式的代數和時, 其反應熱為此二反應熱的代數和。
Horxn = Hoa + Hob + Hoc + …….
28
Hess’s Law
• If we know the following Ho’s
[1]
[2]
[3]
4 FeO(s) + O2(g) 2 Fe2O3(s) Ho=-560 kJ
2 Fe(s) + O2(g) 2 FeO(g) Ho=-544 kJ
4 Fe(s) + 3 O2(g) 2 Fe2O3(s) Ho=-1648 kJ
• For example, we can calculate the Ho for reaction [1] by properly
adding (or subtracting) the Ho’s for reactions [2] and [3].
• Notice that reaction [1] has FeO and O2 as reactants and Fe2O3 as a
product. (2x[-2]) + [3] = [1]
– Arrange reactions [2] and [3] so that they also have FeO and O2 as
reactants and Fe2O3 as a product.
2x
• Each reaction can be doubled, tripled, or multiplied by half, etc.
• The Ho values are also doubled, tripled, etc.
• If a reaction is reversed the sign of the Ho is changed.
[-2] 2 (2 FeO(g) 2 Fe(s) + O2(g)) Ho=2x (-(-544
[3]
4 Fe(s) + 3 O2(g) 2 Fe2O3(s) Ho=-1648 kJ
[1]
kJ) )
4 FeO(s) + O2(g) 2 Fe2O3(s) Ho=-560 kJ
29
Hess’s Law
Example 15-7: Given the following equations and
Ho values
[1] 2N2(g) + O2(g) 2 N2O(g) Ho= 164.1 kJ
[2] N2(g) + O2(g) 2 NO(g) Ho= 180.5 kJ
[3] N2(g) + 2 O2(g) 2NO2(g) Ho= 66.4 kJ
calculate Ho for the reaction below.
N2O (g) + NO2(g) 3 NO(g) Ho= ?
½ [-1] + 3/2 [2] + ½ [-3]
½ [-1]
N2O(g) N2(g) + ½ O2(g)
Ho= ½ x (-164.1) kJ
3/2 [2] 3/2 N2(g) + 3/2 O2(g) 3 NO(g) Ho= 3/2 x (180.5) kJ
½ [-3]
NO2(g) ½ N2(g) + O2(g)
Ho= ½ (-66.4) kJ
N2O (g) + NO2(g) 3 NO(g)
Ho= (-82.05) + 270.75 + (-33.2)
= 155.5 kJ (endothermic reaction)
30
Hess’s Law
Horxn = nHofproducts - Hofreactants
31
Hess’s Law
Example 15-8: Calculate the H o298 for the
following reaction. (Appendix 4, A20)
C3H8(g) + 5 O2(g) 3 CO2(g) + 4 H2O (l)
Horxn = [3 HofCO2(g) + 4 HofH2O(l)]-[HofC3H8(g) + 5 HofO2(g)]
Horxn = [3 (-393.5) + 4 (-286)]-[(-104) + 5 (0.0)]
Horxn = -2220.5 kJ
Exothermic reaction
32
Hess’s Law
Example 15-9: Given the following information,
calculate Hfo for H2S(g).
2 H2S(g) + 3 O2(g) 2 SO2(g) + 2 H2O (l)
Hfo ?
0
Ho298 = -1124 kJ
-296.8 -285.8
Horxn = [2 HofSO2(g) + 2 HofH2O(l)]-[2 HofH2S(g) + 3 HofO2(g)]
-1124 kJ = [2x(-296.8) + 2 (-285.8)]-[2HofH2S(g) + 5 (0.0)]
2 HofH2S(g) = -41.2 kJ
HofH2S(g) = -20.6 kJ
33
Bond Energies 鍵能
• Bond energy is the amount of energy required to
break the bond and separate the atoms in the
gas phase. (一莫耳氣態原子之化學鍵斷裂時吸收的能量即稱
為鍵能)
– To break a bond always requires an absorption of
energy!
A-B(g) + bond energy A(g) + B(g)
H-Cl(g) + 432 kJ/mol H(g) + Cl(g)
34
35
Bond Energies
• Bond energies can be calculated from
otherHo298 values
Example 15-10: Calculate the bond energy for
hydrogen fluoride, HF.
H-F(g) + Bond Energy (BEHF) H(g) + F (g) (atoms NOT ions)
or
H-F(g) H(g) + F (g) Ho298= BEHF
Ho298 = [HofH(g) + HofF(g)]-[ HofHF(g) ]
Ho298 = [218.0 kJ+ 78.99 kJ]-[ -271 kJ]
Ho298 = 568.0 kJ Bond energy for HF
36
Bond Energies
Example 15-11: Calculate the average N-H bond energy
in ammonia, NH3.
NH3(g) N(g) + 3H (g) Ho298= 3 BEN-H
Ho298 = [HofN(g) + 3 HofH(g)]-[ HofNH3(g) ]
Ho298 = [472.7 kJ+ 3x(218) kJ]-[ -46.11 kJ]
Ho298 = 1173 kJ 3 N-H Bond energy for NH3
Average BEN-H = 1173/3
= 391 kJ/mol N-H bonds
37
Bond Energies
• In gas phase reactions Ho values may be related
to bond energies of all species in the reaction.
Ho298 = BEreactants - BEproducts
38
Bond Energies
Example 15-12: Use the bond energies listed in Tables
15-2 and 15-3 to estimate the heat of reaction at
25oC for the reaction below.
CH4(g) + 2 O2(g) CO2(g) + 2 H2O (g)
Ho298 = [4 BEC-H + 2 BEO=O]-[2 BEC=O+4 BEO-H]
Ho298 = [4x(414 kJ) + 2x(498 kJ)]-[2x(741 kJ) + 4x(464 kJ)]
Ho298 = -686 kJ
39
Changes in Internal Energy,
E (內能的改變)
• The internal energy, E, is all of the energy contained
within a substance.
– This function includes all forms of energy such as
kinetic, potential, gravitational, electromagnetic,
etc. (包括所有形式的能量如動能、位能、重力、電磁
能等)
– The First Law of Thermodynamics states that the
change in internal energy, E, is determined by
the heat flow, q, and the work, w.
40
Changes in Internal Energy, E
E = Eproducts – Ereactants
E = q+w
q > 0 if the heat is absorbed by the system
q < 0 if the heat is absorbed by the surroundings
w > 0 if the surrounding do work on the system
w < 0 if the system does work on the surroundings
41
Changes in Internal Energy, E
•E is negative when energy is released by a system
undergoing a chemical or physical change.
– Energy can be written as a product of the process.
C5H12(l) + 8 O2(g) 5 CO2(g) + 6 H2O(l) + 3.516x103 kJ
E = - 3.516x103 kJ
• E is positive when energy is absorbed by a
system undergoing a chemical or physical
change.
– Energy can be written as a reactant of the process.
5 CO2(g) + 6 H2O(l) + 3.516x103 kJ C5H12(l) + 8 O2(g)
E = + 3.516x103 kJ
42
Changes in Internal Energy,
E
Example 15-13: If 1200 joules of heat are added to a
system in energy state E1, and the system does 800
joules of work on the surroundings, what is the :
1. energy change for the system, Esys
E = Eproducts – Ereactants = q+w
E = 1200 J + (-800J)
E sys= +400 J
2. energy change of the surroundings, Esurr
E surr= -400 J
3. energy of the system in the new state, E2
E surr= E2 – E1
E2 = E1 + Esys
43
= E1 +400J
Changes in Internal Energy,
E
•In most chemical and physical changes, the only
kind of work is pressure-volume work.
•Pressure is force per unit area.
force
P = area = F2
d
•Volume is distance cubed.
V = d3
• PV is a work term, i.e., the same units are used for
energy and work.
PV=
F x d3= Fxd (所做的功)
d2
44
Changes in Internal Energy,
E
A system that absorbs heat and does work.
45
Changes in Internal Energy,
E
• Using the ideal gas law PV = nRT, we can look at
volume changes of ideal gases at constant T
and P due to changes in the number of moles of
gas present, ngas.
PV = nRT
P(V)=(ngas)RT
ngas = (number of moles of gaseous products) - (number of
moles of gaseous reactants)
• Work is defined as a force acting through a
specified distance.
w= F x d = -PV = -(ngas)RT
w = -(ngas)RT at constant T and R
46
Changes in Internal Energy,
E
• Consequently, there are three possibilities for
volume changes:
When
Then
1. V2 = V1 PV = 0
ngas = 0
2.
3.
V2 > V1 PV > 0
ngas > 0
V2 < V1 PV < 0
ngas < 0
Examples
CO(g) + H2O(g) H2(g) + CO2(g)
2 mol gas
2 mol gas
Zn(s) + 2HCl(aq) ZnCl2(aq) + H2(g)
3 mol gas
1 mol gas
N2(g) + 3 H2(g) 2 NH3(g)
4 mol gas
2 mol gas
47
Changes in Internal Energy, E
• Consider the following gas phase reaction at
constant pressure at 200oC.
2 NO(g) + O2(g) 2 NO2(g)
3 mol gas
2 mol gas
V2 < V1 thus V<0 and PV<0
w= -PV >0
Works is done on system by surroundings
• Consider the following gas phase reaction at
constant pressure at 1000oC.
PCl5(g) PCl3(g) + Cl2(g)
1 mol gas
2 mol gas
V2 > V1 thus V>0 and PV>0
w= -PV <0
Works is done by the system on the surroundings 48
Relationship of H and E
• The total amount of heat energy that a system can
provide to its surroundings at constant temperature
and pressure is given by
H= E + PV
– which is the relationship between H and E.
H = change in enthalpy of system
E = change in internal energy of system
PV = work done by system
49
Relationship of H and E
• At the start of Chapter 15 we defined
H = qP.
• Here we define H = E + PV.
– Are these two definitions compatible?
• Remember E = q + w.
• We have also defined w = -PV .
– Thus E = q + w = q -PV
• Consequently, H = q- PV + PV = q
– At constant pressure H = qP.
50
Relationship of H and E
• For reactions in which the volume change is very
small or equal to zero.
– For small volume changes
V 0 and PV 0
H = E + PV
H E
– For no volume change,
H = E
51
Relationship of H and E
• Change in enthalpy, H, or heat of reaction is
amount of heat absorbed or released when a
reaction occurs at constant pressure.
• The change in energy, E, is the amount of heat
absorbed or released when a reaction occurs at
constant volume.
• How much do the H and E for a reaction differ?
– The difference depends on the amount of work performed
by the system or the surroundings.
52
Relationship of H and E
Bomb calorimeter
53
Relationship of H and E
Example 15-14: In Section 15-5, we noted that Ho = -3523
kJ/mol for the combustion of n-pentane, n-C5H12.
Combustion of one mol of n-pentane at constant
pressure releases 3523 kJ of heat. What are the values of
the work term and E for this reaction?
C5H12(l) + 8 O2(g) 5 CO2(g) + 6 H2O(l) Horxn= - 3523 kJ
8 mol gas
5 mol gas
Horxn= - 3523 kJ/mol T=298K
w= -PV = -(ngas)RT
ngas = 5-8 mole = -3 mole
w= -(-3ml)(8.314 J/mol K)(298)
= 7433 J = 7.433 kJ
H = E + PV E= H-PV
E = -3523 kJ – (7.433 kJ)
= 3516 kJ
54
Spontaneity of Physical and
Chemical Changes
• Spontaneous changes happen without any
continuing outside influences.
– A spontaneous change has a natural direction.
(product-favored)
• For example the rusting of iron occurs
spontaneously. (鐵生鏽)
– Have you ever seen rust turn into iron metal
without man made interference?
• The melting of ice at room temperature occurs
spontaneously.
– Will water spontaneously freeze at room
temperature?
55
The Two Aspects of Spontaneity
• Two factors affect the spontaneity of any
physical or chemical change:
– Exothermic
• An exothermic reaction does not ensure
spontaneity.
– For example, the freezing of water is exothermic
but spontaneous only below 0oC.
– Increase in the dispersal of energy and matter
• An increase in disorder of the system also does
not insure spontaneity.
– It is a proper combination of exothermicity
and disorder that determines spontaneity.
56
Entropy, S (熵)
•Entropy is a measure of the disorder or randomness of
a system. 熵用以測量一個系統的亂度
–The greater the energy dispersal in a system, the
higher is its entropy
•As with H, entropies have been measured and
tabulated in Appendix K as So298.
•When:
– S > 0 disorder increases (which favors
spontaneity).
– S < 0 disorder decreases (does not favor
spontaneity).
57
Entropy, S
Suniverse = Ssystem + Ssurroundings >0
• In general for a substance in its three states
of matter:
Sgas > Sliquid > Ssolid
58
• Entropy increase (Ssysytem>0), When
–
–
–
–
–
–
Temperature increase
Volume increase
Mixing of substance
Increasing particle number
Molecular size and complexity
Ionic compounds with similar formulas but different
charges
Example
Without doing a calculation, predict whether the entropy
change will be positive or negative
a) C2H6(g) +7/2 O2(g) 3H2O(g) + 2 CO2(g)
b) 3C2H2(g) C6H6(l)
c) C6H12O6(s) + 6 O2(g) 6 CO2(g) + 2 H2O(l)
a) S0>0
b) S0<0
c) S0>0
d) Hg(l)< Hg(s) <Hg(g)
d) Hg(l), Hg(s), Hg(g)
e) C2H6(g), CH4(g) , C3H8(g)
e) CH4(g)< C2H6(g)< C3H8(g)
f) CaS(s), CaO(s)
f) CaO(s)< CaS(s)
59
Entropy, S
• The Third Law of Thermodynamics states,
“The entropy of a pure, perfect, crystalline
solid at 0 K is zero.”熱力學第三定律是指當一個系統
趨近於絕對溫度零度時,系統的熵變化率乃零。
• This law permits us to measure the absolute
values of the entropy for substances.
– To get the actual value of S, cool a substance to
0 K, or as close as possible, then measure the
entropy increase as the substance heats from 0
to higher temperatures.
– Notice that Appendix K has values of S not S.
60
• Entropy changes for reactions can be
determined similarly to H for reactions.
So298 = n nSoproducts - n nSoreactants
Example 15-15: Calculate the entropy change for the
following reaction at 25oC. Use appendix K.
2 NO2(g) N2O4(g)
So298 = nSoproducts - nSoreactants
n
n
= SoN2O4(g) - 2SoNO2(g)
= (304.2 J/mol K) - 2x(240.0 J/mol k)
= -175.8 J/mol k
• The negative sign of S indicates that the system is
more ordered.
• If the reaction is reversed the sign of S changes.
– For the reverse reaction So298= +0.1758 kJ/mol K
• The + sign indicates the system is more disordered.61
Entropy, S
• Example 15-16: Calculate So298 for the reaction
below. Use appendix K.
3 NO(g) N2O(g) + NO2(g)
So298 = SoN2O(g) + SoNO2(g) – 3 SoNO(g)
= [219.7 + 240.0 – 3x(210.4)] J/mol K
= -172.4 J/mol K (-1.724 kJ/mol K)
• Changes in S are usually quite small compared to
E and H.
– Notice that S has units of only a fraction of a kJ while
E and H values are much larger numbers of kJ.
62
Example 15-15: Calculation of ΔSo for a phase change
Use the values of standard molar entropies in Appendix K to
calculate the entropy change for the vaporation of one
mole of bromine at 25oC.
Br2(l) Br2(g)
The values ΔSo from Appendix K:
Br2(l)
Br2(g)
So, J/mol•k 152.2
245.4
ΔSo= ΣnSoproducts - Σ nSoreactants
= 1(245.4) – 1(152.2)
= 93.2 J/mol•K
63
Example 15-16: Calculation of ΔSorxn
Use the values of standard molar entropies in Appendix K to
calculate the entropy change at 25oC and one
atmosphere pressure for the reaction . Do you think the
reaction is spontaneous?
N2H4(l) + 2H2O2(l) N2(g) + 4H2O(g) ΔHorxn= -642.2 kJ/mol rxn
The values ΔSo from Appendix K:
N2H4(l)
H2O2(l)
N2(g)
So, J/mol•k 121.2
109.6
191.5
H2O(g)
188.7
ΔSorxn= ΣnSoproducts - Σ nSoreactants
= [1(191.5)+4(188.7)] – [1(121.2)+2(109.6)]
= 605.9 J/mol•K
Δ H<0 exothermic reaction
Δ S >0
Spontanoue reaction
64
The Second Law of
Thermodynamics
• The second law of thermodynamics states, “In
spontaneous changes the universe tends
towards a state of greater disorder.”熱力學第二定
律指自發性的反應傾向最大亂度
• Spontaneous processes have two requirements:
1.The free energy change of the system must be
negative. 系統內自由能的改變為負值
2.The entropy of universe must increase. 亂度必增加
• Fundamentally, the system must be capable of doing
useful work on surroundings for a spontaneous process
to occur.
65
Free Energy Change, G,
and Spontaneity
•In the mid 1800’s J. Willard Gibbs determined the
relationship of enthalpy, H, and entropy, S, that
best describes the maximum useful energy
obtainable in the form of work from a process at
constant temperature and pressure.
– The relationship also describes the spontaneity of
a system.
• The relationship is a new state function, G,
the Gibbs Free Energy.
G= H - TS (at constant T & P)
66
• The change in the Gibbs Free Energy, G, is a
reliable indicator of spontaneity of a physical
process or chemical reaction.
– G does not tell us how quickly the process
occurs.
• Chemical kinetics, the subject of Chapter 16, indicates
the rate of a reaction.
• Sign conventions for G.
– G > 0
– G = 0
– G < 0
reaction is nonspontaneous
system is at equilibrium
reaction is spontaneous
• 化學反應的G>0,表示反應產生的能量不足以克服阻抗,反應不能夠自然
地發生。
• 化學反應的G=0,表示反應處於平衡狀態,正向與逆向反應的驅力相等。
• 化學反應的G<0,表示該反應釋放的能量足以克服外圍的阻抗,反應會順
利地朝產物的方向進行,指示這是一個自發性的反應。
H越小越有助於G<0,也就是反應放熱越多越有助於G<0。
S越大越有助於G<0,也就是產物的亂度變得越大越有助於G<0。
這並不意味,吸熱反應或減小亂度的反應不會自然發生。一個反應會不
會自然發生,完全看G是不是小於0。
67
Free Energy Change, G,
and Spontaneity
• Changes in free energy obey the same type of
relationship we have described for enthalpy, H,
and entropy, S, changes.
Go298 = n nGoproducts - n nGoreactants
68
Free Energy Change, G,
and Spontaneity
• Example 15-17: Calculate Go298 for the reaction in
Example 15-8. Use appendix K.
C3H8 (g) + 5 O2(g) 3 CO2(g) + 4 H2O(l)
Go298 = nGoproducts - nGoreactants
Go298 = [3 GofCO2(g) + 4 GofH2O(l)) ]-[GofC3H8(g) + 5GofO2(g)]
= {[3x(-394.4) + 4x(-273.3)]-[(-23.49)+5(0)]} kJ/mol
= - 2108.9 kJ/mol
Go298 < 0, so the reaction is spontaneous at standard
state conditions.
• If the reaction is reversed:
Go298 > 0, and the reaction is nonspontaneous at
standard state conditions.
69
The Temperature
Dependence of Spontaneity
• Free energy has the relationship G = H -TS.
• Because 0 ≤ H ≥ 0 and 0 ≤ S ≥ 0, there are four
possibilities for G.
H
S
G
Forward reaction spontaneity
<0
>0
<0
Spontaneous at all T’s.
<0
<0
T dependent
Spontaneous at low T’s.
>0
>0
T dependent
Spontaneous at high T’s.
>0
<0
>0
Nonspontaneous at all T’s.
70
The Temperature
Dependence of Spontaneity
71
The Temperature
Dependence of Spontaneity
Example 15-18: Calculate So298 for the following reaction.
In example 15-8, we found that Ho298= -2219.9 kJ, and
in Example 15-17 we found that Go298= -2108.5 kJ.
C3H8 (g) + 5 O2(g) 3 CO2(g) + 4 H2O(l)
Go = Ho - TSo
So =
TSo = Ho- Go
[(-2219.9) – (-2108.50)] kJ
o
S =
298 K
Ho - Go
T
So = -0.374 kJ/K =-374 J/k
So298 = -374 J/K which indicates that the disorder of the
system decreases .
For the reverse reaction,3CO2(g)+ 4H2O(g) C3H8(g) + 5 O2(g)
So298 = +374 J/K which indicates that the disorder of the
72
system increases .
The Temperature
Dependence of Spontaneity
Example 15-18: Use thermodynamic data to estimate
the normal boiling point of water. And What is the
percent error
H2O (l) H2O(g)
Because this is an equilibrium process G=0
Thus H = TS and T = H/S
o
Assume
S@BP
S
o
rxn
Assume H@BP H 298
o
o
o
S
=
S
o
o
o
(g) - S H2O(l)
2
rxn
H
O
H = H H2O(g) - H H2O(l)
o
S
o
rxn = [188.7 – 69.91]
H = [(-241.8) – (-285.8)]
o
S
o
o
rxn = 118.8 J/k
H = 44.0 kJ @ 25 C
= 0.1188 kJ/k
T = H/S Ho/So = 44.0 kJ/0.1188 kJ/K = 370 K
370 K – 273 K = 97 oC
% error = (370-373) K x100% = -0.8% error
73
373 K