PowerPoint **
advertisement

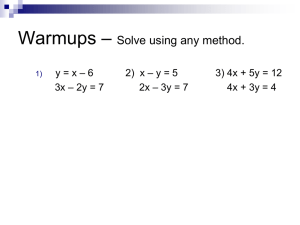
Chapter Two Polar Reaction Under Basic Conditions a. Substitution and Elimination at C(sp3)-X σ bonds b. Addition of Nuclephiles to Electrophilic π bonds c. Substitution at C(sp2)-X σ bonds d. Base-promoted Rearrangements Nuclephility and Basicity SN 2 Nu- Good nuclephiles and good bases Unhindered RO-, R2N-, R3N, RC≡C-, Cl- SN 1 R- Good nuclephiles and poor bases Br-, I-, R2S, RS-, R3P, malonate anion, R2CuLi B- E2 E1 Poor nuclephiles and good bases (bulky) t-BuO-, i-Pr2NLi(LDA), R3N, (TMS)2NK, i-Pr2NEt, t-BuLi TMS: Trimethylsilane SN2, E2: basic condition SN1, E1: acidic condition Substitution by the SN2 Mechanisms R S a. Back attack SP2 C and 3o C can’t undergo SN2 b. Sterospecific c. Only 1o and 2o C(sp3) undergo SN2 SNAr How to retent the configuration? S S Solvent: Polar Aprotic DMSO, DMF, Acetone, THF, MeCN, EA… Polar solvent can stabilize the intermediate. Aprotic solvent can avoid H+ react with Nu-. Loss Configurational Purity by Nuclephilic Substitution The leaving group is α or β to a carbonyl group. Substituted group is good nuclephile, also good leaving group. Elimination by the E2 Mechanisms β hydrogen, Good base, 3o C Stereochemistry of E2 Newman projection Sawhore projection Please draw the structure of product. b. a. E2 c. E2 d. Syn Elimination If the base were part to the substrate, the acidic hydrogen be removed in an intramolecular reaction(syn elimination). Hofmann Elimination Syn elimination major E1cb and 1,3-Elimination E1cb: β hydrogen is particularly acidic(carbonyl) and leaving group is poor(-OH, -OR) hemiacetal 1,3-Elimination(decarboxylation) CH2COOH carbonyl Substitution by the Elimination-Addition Reaction Nu: -OMe E+: carbonyl group, Br No SN2 due to the steric hindrance. Leaving group: Br, β hydrogen Elimination-Addition Reaction better electrophile than carbonyl group(steric) Please draw the reasonable mechanisms of this reaction Exercises Please draw the mechanisms of following reaction a. e. CO2 b. c. CO2 d. α-Elimination: Generation of Carbene Defination: A carbene is a divalent carbon species link to two adjacent groups by covalent bonds, possessing two nonbonded electrons and six valence electrons. Preparation of carbenes a. b. c. d. - + Reaction of Carbene Exercises Please draw the mechanisms of following reaction a. b. c. d. e. Polar Reaction Under Basic Conditions a. Substitution and Elimination at C(sp3)-X σ bonds b. Addition of Nuclepphiles to Electrophilic π bonds c. Substitution at C(sp2)-X σ bonds d. Base-promoted Rearrangements Carbonyl Group Under basic conditions, carbonyl compounds are electrophilic at carbonyl C and nuclephilic at α C’s. R is donating group Stabilize the carbocation decrease the reactivity Arrange the stabilities and reactivities of carbonyl compounds as follow. Carbonyl Group As Electrophile a. M-Nu (R-MgBr, NaBH4, LiAlH4, R2CuLi) b. Amines as nuclephiles (Please draw the mechanism) c. Water and alcohols as nuclephiles under basic conditions. base Carbonyl Group As Nuclephiles (Aldol Reaction) Aldol reaction: Enolates react with ketones and aldehydes. Draw mechanisms for the following aldol reactions Michael Addition Michael addition: The 1,4-(conjugated) addition of a carbon nuclephile to an α, β-unsaturated carbonyl system is referred to as Michael addition. Draw mechanisms for the following reactions d. a. b. e. c. Baylis-Hillman Reaction and Robinson Annulation a. Baylis-Hillman reaction: An acrylate ester reacts with an aldehyde in the presence of an amine or phosphine catalyst. b. Robinson annulation Polar Reaction Under Basic Conditions a. Substitution and Elimination at C(sp3)-X σ bonds b. Addition of Nuclephiles to Electrophilic π bonds c. Substitution at C(sp2)-X σ bonds d. Base-promoted Rearrangements Substitution at Carbonyl C Draw mechanisms for the following reaction and explain why carbonyl acid can’t undergo similar reaction Reduction of aldehyde, ketone or ester. Organometallic reagents as Nu-(RMgBr, R2CuLi…) Substitution at Carbonyl C Claisen condensation: An ester enolate is condensed with a ketone, aldehyde, or ester. Dieckmann condensation: An intramolecuar version of the Claisen condensation Draw mechanisms for the following reactions Substitution at Alkenyl C and Aryl C(SNAr) α, β-Unsaturated carbonyl compounds with a leaving group in the β position are susceptible to addition-elimination reactions. SNAr: Aromatic compounds that are substituted with electron-withdrawing groups undergo nuclephilic aromatic substitution. Favor Unfavor Nuclephilic Aromatic Substitution(SNAr) Explain the results which was showed below A A Draw mechanisms for the following reaction B B Substitution at Aryl C(SNAr) Aryl halides undergo substitution reactions with very strong base such as –NH2, terbutyl lithium. Why alkenyl halides such as CH3CBr=ChCH3 don’t undergo substitution upon treatment with a strong base(-NH2)? Ans: ring strain. Sandmeyer reaction Nu: CuX, H2O, X-, CN-, H3PO2 Ex Polar Reaction Under Basic Conditions a. Substitution and Elimination at C(sp3)-X σ bonds b. Addition of Nuclepphiles to Electrophilic π bonds c. Substitution at C(sp2)-X σ bonds d. Base-promoted Rearrangements Migration from C to C Favorskii rearrangemet Please draw the mechanisms Diazomethane(CH2N2) reacts with ketones(R2C=O) to insert CH2 unit between C=O and R Baeyer-Villiger rearrangement Wolf rearrangement Please draw the mechanisms Migration from C to O or N Baeyer-Villiger rearrangement base Curtius rearrangement (acyl chloride to amine) Hofmann rearrangement (amide to amine) Please draw the mechanisms of Hofmann rearrangement The Swern Oxidation 1o alcohol to aldehyde; 2o alcohol to ketone Mechanism The Mitsunobu Reaction A 2o alcohol and a carboxylic acid are converted to an ester. A poor leaving group is converted to an excellent leaving group. S Mechanism R Draw mechanisms for the following reactions a. b. c. d. B e. Draw mechanisms for the following reactions a. f. b. g. h. c. d. i. e. Thanks For Your Attention