Carbonyl chemistry - MrFisherChemistry
advertisement
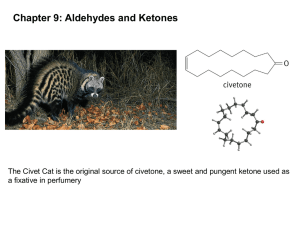
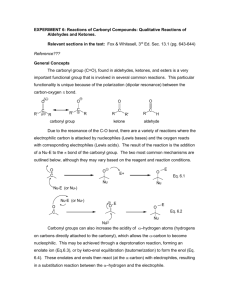
Carbonyl chemistry -Production of carbonyl compounds -Oxidation and Reduction reactions -Further oxidation reactions. Formation of carbonyl compounds • Starting with an alcohol, react with an oxidising agent (such as acidified potassium dichromate) • Propan-1-ol + [O] Propanal +water The carbonyl functional group The bond is polar due to the difference in electronegativity. PLANAR WITH BOND ANGLES OF 120° ORBITAL OVERLAP NEW ORBITAL Aldehydes vs. Ketones • Aldehydes have the carbonyl group at the end of a carbon chain (joined to only one carbon) H CH3 C=O H C=O H • Ketones have the carbonyl group bonded to two carbons. CH3 C2H5 C=O CH3 C=O CH3 Naming aldehydes/ketones Aldehydes C2H5CHO propanal Ketones CH3COCH3 propanone CH3CH2COCH3 butanone CH3COCH2CH2CH3 pentan-2-one CH3CH2COCH2CH3 pentan-3-one C6H5COCH3 phenylethanone Identification of carbonyls • IR spectroscopy shows a strong peak around 1400-1600 cm-1 Oxidation of carbonyl • Reacting an aldehyde with an oxidising agent (acidified dichromate ions) produces a carboxylic acid. • Propanal + [O] Propanoic acid +water Reduction of carbonyls • Carbonyl groups can also be reduced. • Reduction agent used is Sodium tetrahydroborate(III) (also called Sodium Borohydride) • This is classed as a weak reducing agent. Reduction of carbonyls • Propanal is reduced to a primary alcohol by sodium borohydride. • Propanal + 2[H] Propan-1-ol Reduction continued • NaBH4 reacts with ketones also. • Propanone + 2[H] propan-2-ol Reduction explained • The BH4 ion is acting as a source of hydride ions (:H-) » Hydride ions being a negatively charged hydrogen ion containing a lone pair of electrons • The mechanism is an example of nucleophilic addition. – H- ion is attracted to the δ+C of the carbonyl, forming a new C-H bond – The resulting O- ion forms a dative covalent bond using the H+ from a water molecule Reduction reaction mechanism • Propanal + 2[H] propan-1-ol Carbonyl questions • Write equations + mechanisms for the reduction of: – Butanal – 2-methylhexan-3-one • Define the term nucleophile • Show the mechanism for the reaction between butanone and NaBH4 Carbonyl chemistry 2 chemical tests on carbonyls -How to detect a carbonyl group -Aldehyde or ketone? ILPAC experiment 8.4 • Using the instruction sheets, follow the method for experiment 8.4 A and 8.4 B • DO NOT DO EXPERIMENT C • Make full and detailed notes on what you observe during the reactions. Condensation reactions • We can detect the presence of a carbonyl group using 2,4-dinitrophenylhydrazine (abbreviated to 2,4-DNP or 2,4-DNPH). • 2,4-DNPH is also called Brady’s reagent when prepared with methanol and sulfuric acid. Using 2,4-DNPH • When Brady’s reagent is added to an aldehyde or ketone, a yellow or orange precipitate is formed. • The precipitate, called a 2,4dinitrophenylhydrazone derivative confirms the presence of a carbonyl functional group. Aldehyde or ketone • Aldehydes and ketones can be distinguished from each other by using Tollen’s reagent Ammoniacal silver nitrate • Tollen’s reagent is a weak oxidising agent, the carbonyl group is oxidised to form a carboxylic acid. Tollen’s reagent. • Silver ions are reduced to silver metal • Ag+(aq) + e- Ag(s) • Aldehyde + [O] Carboxylic acid Carbonyl questions 2 • The carbonyl compounds CH3COCH3 and CH3CH2CHO are structural isomers. – Name these compounds – State the reagents used and observations made to prove the presence of a carbonyl group in these compounds – State the reagents and observations made to distinguish between the two chemicals. Carbonyl chemistry 3 Identifying a carbonyl compound -Determining experimentally an unknown carbonyl compound ILPAC 8.5 • Using the instruction sheets given last lesson, carry out experiment 8.5, parts A and B • Complete a detailed method for identifying a carbonyl compound – Purifying by recrystallisation -> melting point determination -> compare melting point to known values. Carbonyl questions 3 • Explain how you would use the solid obtained from the reaction of an aldehyde with 2,4DNPH to prove the original aldehyde was butanal. • The reaction of 2,4-DNPH with ethanal gives water as a product. Suggest the type of reaction taking place.