dozen
advertisement

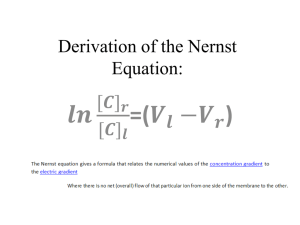
A DOZEN roses = 12 roses A PAIR of shoes = 2 shoes a DOZEN eggs = 12 eggs a REAM of paper = 500 sheets A GROSS of pencils = 144 pencils A SIX –PACK of beer = 6 cans TRIO QUARTET When you have a dozen of something, no matter what you are talking about, you have 12 of that thing. -A dozen donuts - a dozen eggs -a dozen cars, -a dozen chemistry problems are all 12 of that material. The mole is the exact same concept. You can have: -a mole of eggs - a mole of donuts - a mole of cars - a mole of chemistry problems. However a mole is MUCH bigger than 12 ...it is 602 sextillion. Obviously a mole of chemistry problems is way too many problems. The mole is typically used for counting or grouping very very small things, like atoms a MOLE (mol) 1. The mole is an SI unit that allows us to count # of: - Atoms - molecules - formula units Representative particles without actually seeing them 2. 1 mol = 6.02 x representative particles 23 10 3. The number is called “Avogadro’s number” in honor of the Italian scientist Amadeo Avogadro Avogadro’s number 1 mole of anything = 6.02 x Atoms or Molecules or FUNs 23 10 MOLE DAY 4. REPRESENTETIVE particles: 1. ATOMs (elements on the PT) 2. MOLECULES (_______compounds covalent – all elements are non metals) EX: CO2, NO2, C12H22O11 ionic 3. Formula Units (_____compounds – at least one element must be a metal) EX: NaCl, CuCl2, FeO A DOZEN MASS of a dozen limes = MASS of a dozen cars A MOLE CARBON MASS of a mole carbon COPPER = MASS of a mole copper 6.02 x 1023 C atoms = 6.02 x1023 Cu atoms HOW DO YOU DETERMINE THE MASS OF A MOLE ? MOLAR MASS – the mass of 1 mole of any pure substance (element and compound) Molar Mass (MM) = Atomic mass (AM) MM units - g/mol AM - Atomic Mass units - amu (atomic mass units) EX: Mn - 54.94 amu = 54.94 g/mol EXAMPLES: 1. 1 mole of Al : AM 26.98 g = MM 26.98 g/mol 2. 1 mole of CO2: # of atoms = # of moles AM (C) = 12.011 amu = MM AM (O) = 16.00 amu x 2 = MM 12.011 + 2 (16.00) = 44.01 g/mole 3. 1 mole of Cu(NO2)2: AM (Cu) 63.546 amu = MM g/mole AM (N) 14 x 2 amu = MM g/mole AM (O) 16 x 4 amu = MM g/mole 63.546 + 2(14) + 4 (16) = 155.5 g/mole