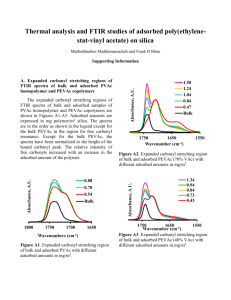
MOFSpec2012_final
advertisement
Vibrational Shift of Adsorbed CO2 within a Metal-Organic Framework Outline • Motivation: CO2 capture • System: Metal-Organic Frameworks • Data: Unusual blue shift of adsorbed CO2 n3 mode Room-temperature sidebands Low-temperature bands reveal 2nd configuration Motivation • Carbon capture – Separate carbon dioxide from exhaust gases – Emissions reduction accompanying switch to clean energy sources • Natural gas purification – Separate CO2 from methane (CH4) – Improve energy density of fuel, decrease pipe corrosion • Current CO2 separation methods are costly – Harmful materials – High energy costs for regeneration – A better way? http://www.nma.org/ccs/carboncaptu Metal-Organic Frameworks Metal ions linked by organic chains Very low density Crystalline and “tunable” Vast number of possible structures Large voids, voids of ~ 10 – 20 Å for molecular storage and separation Complex unit cell makes computation modeling challenging Significant van der Waals interactions MOF-74 Honeycomb structure Metal-oxide clusters linked by Benzene rings Unsaturated metal ion acts as primary binding site for CO2 2+ 2.4 Å Diffraction indicates CO2 is nearly linear H. Wu et al. J. Phys. Chem. Lett., 1(13):1946–1951, 2010. MOF-74 Isostructural Series Same structure, different metal Mg-MOF-74, Mn-MOF-74, Fe-MOF-74 ,Co-MOF-74, Zn-MOF-74 http://legacy.owensboro.kctcs.edu/gcaplan/bio/Notes/BIO%20Notes%20C%20intro%20chem.htm MOF-74 Selective Binding Binding energy in Mg-MOF-74 ~ 40 - 50 kJ/mol Binding energy in other MOF-74 at least 7 kJ/mol less Difference is likely due to direct electrostatic interaction via shorter Mg – O bond Binding energy for CH4 in MOF-74 ~ 20 kJ/mol Difference between CO2 and CH4 mainly attributed to CO2 quadrupole moment Caskey et al. J. Am. Chem. Soc., 130,10870, (2008). H. Wu et al. J. Am. Chem. Soc., 131, 4995 (2009). Park et al. Phys. Chem. Lett. 3, 826 (2012). Yao et al. Phys. Rev. B. 85, 64302 (2012). Diffuse Reflectance Spectroscopy • Light bounces around within powder sample • Very long path length enhances absorption signal Diffuse Reflectance Spectroscopy: Cryostat Assembly Rev. Sci. Instr. 77, 093110 (2006) Absorbance (Arb. Units) n3 mode of adsorbed CO2 Mg Mn Fe Co Zn 2300 2320 2340 2360 -1 Frequency (cm ) 2380 2400 Vibration of adsorbed H2 Absorbance Fe Co Mn Mg Zn 4050 4100 Frequency (cm ) -1 4150 Absorbance (Arb. Units) n3 mode of adsorbed CO2 Mg Mn Fe Co Zn 2300 2320 2340 2360 -1 Frequency (cm ) 2380 2400 Side Bands: Translational/Librational 000 →001 Absorbance (Arb. Units) 0.1 2250 010 →011 C13 2300 2350 2400 -1 Frequency (cm ) 2450 Librational Motion MOF-74 2+ H. Wu et al. J. Phys. Chem. Lett., 1(13):1946–1951, 2010. Temperature Dependence New band emerges below 150 K 251 K 1.0 163 K 0.8 104 K 14 ΔEB = 0.7 ± 0.1 kJ/mol Degeneracy ratio of ~ 2 44 K 34 K Frequency (cm ) 12 16 -1 1000/T (K ) 54 K 2360 -1 0.4 0.0 10 65 K 2350 0.6 0.2 75 K 2340 Room temperature peaks too broad to resolve Ln(A1/A2) Absorbance (Arb. Units) 0.1 2370 18 vdW-DF2 Theory Calculations Y. Yao et al. Phys. Rev. B, 85, 064302 ( 2012). Predicts sites 2.96 and 3.09 Å away from metal with 0.8 kJ/mol energy difference Combination modes compared to Hitran Data Absorbance (Arb. Units) n3 000 → 001 n3 + Fermi resonance 000 → 101 and 000 → 021 2400 0.01 0.05 0.05 2300 n3 + 2 x Fermi resonance 000 → 201, 041, 121 3600 3800 -1 Frequency (cm ) 4800 5000 5200 Conclusion CO2 in MOF-74 • Mg version n3 mode unique in showing blue shift • All other modes show red shift • Evidence for CO2 librational/translational motion • Evidence for a 2nd “nearly degenerate” adsorbed CO2 configuration Undergrad Students Michael Friedman Jordan Gotdank Jesse Hopkins Brian Burkholder Ben Thompson Chris Pierce Jennifer Schloss