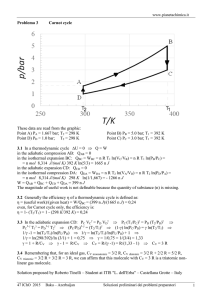
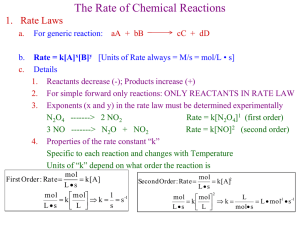
Chapter 4 Student Presentation
advertisement

Chapter 4 Aqueous Reactions and Solution Stoichiometry Chapter Objectives • Metathesis reactions (double replacement) – precipitation reactions – acid-base reactions – redox reactions • Single Replacement reactions (displacement) – activity series • Molar concentration • Solution Stoichiometry – titration – indicators General Properties of Aqueous Solutions • Homogeneous mixtures of two or more pure substances. • The solvent in greatest abundance. • All other substances are solutes. • If water is the solvent the solution is aqueous Electrolytic Properties • whether or not solutions conduct electricity. • If ions form in solution, the substance is an electrolyte and the solution conducts electricity. e.g. NaCl. • If no ions form, the substance is a nonelectrolyte. e.g. sucrose. • Ions form in solution through the process of dissociation. Dissociation • Solid does not exist as a well-ordered crystal. • Each ion surrounded by a shell of water molecules. – cations have oxygen end of water pointing in. – anions have hydrogen end of water pointing in. • Ions move to cause electric current to flow through solution. • Electrolytes - Soluble ionic compounds • Non-electrolytes Molecular compounds Electrolytes • A strong electrolyte dissociates completely when dissolved in water. – ionic compounds, many acids & bases. • A weak electrolyte only dissociates partially when dissolved in water. – weak acids and bases. Solvation Equations • A substance dissolves in a solvation reaction: – sugar is a non-electrolyte: C12H22O11 (s) → C12H22O11 (aq) • soluble ionic compounds undergo dissociation in solution; break up into constituent ions: NaCl (s) → Na1+(aq) + Cl1-(aq) (this represents both solvation and dissociation) • to write a dissociation equation for any ionic compound identify the cation and anion, then write the balanced equation: • Al2(SO4)3 (s) contains Al3+ and SO42- ions. • When it dissociates you get 2 Al3+ and 3 SO42ions: Al2(SO4)3 (s) → 2 Al3+(aq) + 3 SO42-(aq) • (make sure the equation is balanced) • Write dissociation equations for the ionic substances in questions 4.15 and 4.16 Precipitation Reactions • When a mixture of soluble ions forms a compound that is insoluble, a precipitate is formed. • Pb(NO3)2 (aq) + 2 KI(aq) PbI2 (s) + 2 KNO3 (aq) Solubility Guidelines for Ionic Compounds • Solubility is the amount of a substance that can be dissolved in a given quantity of solvent at that temperature. • Measured in g/100g H2O, or mol/L • Solubility < 0.01 mol/L is insoluble. • Experimental observations have led to empirical guidelines for predicting solubility. Solubility of Selected Ions (soluble > 0.1 mol/L) Anion any any nitrate ion (NO31-) chlorate ion (ClO31-) acetate ion (CH3COO1-) chloride (Cl1-), bromide (Br1-), iodide (I1-) Sulfate (SO42-) + Cation + group 1 metal ion (Li1+, Na1+, K1+, Rb1+, Cs1+) + ammonium ion (NH41+) + any = Solubility is soluble + + + + is is is is + + Sulfide (S2-) + Hydroxide (OH1-) + + Phosphate (PO43-) Carbonate (CO32-) Sulfite (SO32-) Oxide (O2-) + Ag1+ any other cation Ag1+, Pb2+, Hg22+, or Cu1+ Ag1+, Pb2+, Hg22+, Hg2+ or Cu1+ any other cation Ca2+, Sr2+, Ba2+, Ra2+, Ag1+, Pb2+, Hg22+ Hg2+ any other cation group 1 & 2 elements, NH41+ any other cation group 1 elements & NH41+, Ca2+, Sr2+, Ba2+ any other cation group 1 elements & NH41+ any other cation is soluble is soluble not soluble soluble not soluble not soluble soluble is not soluble is soluble is is is is soluble not soluble soluble not soluble is soluble is not soluble Predicting Solubility • Na2S – soluble, because Na is an alkali metal (group 1) • NH4Cl – soluble, because the NH41+ ion is soluble with any anion • CuNO3 – soluble, because NO31- is soluble with any cation • AgCH3COO – insoluble • Mg(CH3COO)2 – soluble • PbCl4 – soluble; in this compound the Pb is 4+ • PbCl2 – insoluble; in this compound the Pb is 2+ Predicting Solubility • • • • • • • • • • KCl NaNO3 AgCl Al2(SO4)3 FeS Ca3(PO4)2 BaSO4 Pb(ClO3)2 (NH4)2S PbCl2 • Complete questions 4.19 and 4.20 Metathesis (Exchange) Reactions • Metathesis comes from a Greek word that means “to transpose” AgNO3 + KCl AgCl (s) + KNO3 (aq) Metathesis (Exchange) Reactions • Metathesis comes from a Greek word that means “to transpose” • It appears the ions in the reactant compounds exchange, or transpose, ions AgNO3 + KCl AgCl (s) + KNO3 (aq) Metathesis (Exchange) Reactions • Metathesis comes from a Greek word that means “to transpose” • It appears the ions in the reactant compounds exchange, or transpose, ions AgNO3 + KCl AgCl + KNO3 Metathesis (Exchange) Reactions • Metathesis comes from a Greek word that means “to transpose” • It appears the ions in the reactant compounds exchange, or transpose, ions • always indicate the phase of the substance, where known AgNO3 (aq) + KCl (aq) AgCl (s) + KNO3 (aq) • most are reactions of ionic compounds Chemical Equations of Metathesis Reactions Molecular Equation The molecular equation lists the reactants and products in their molecular form. AgNO3 (aq) + KCl (aq) AgCl (s) + KNO3 (aq) Complete Ionic Equation • All strong electrolytes are dissociated into their ions. • This more accurately reflects the species that are found in the reaction mixture. Ag+ (aq) + NO3- (aq) + K+ (aq) + Cl- (aq) AgCl (s) + K+ (aq) + NO3- (aq) Net Ionic Equation • To form the net ionic equation, cross out anything that does not change from the left side of the equation to the right. Ag+(aq) + NO3-(aq) + K+(aq) + Cl-(aq) AgCl (s) + K+(aq) + NO3-(aq) Net Ionic Equation • To form the net ionic equation, cross out anything that does not change from the left side of the equation to the right. • The only things left in the equation are those things that change (i.e., react) during the course of the reaction. Ag+(aq) + Cl-(aq) AgCl (s) Net Ionic Equation • To form the net ionic equation, cross out anything that does not change from the left side of the equation to the right. • The only things left in the equation are those things that change (i.e., react) during the course of the reaction. • Those things that didn’t change (and were deleted from the net ionic equation) are called spectator ions. Ag+(aq) + NO3-(aq) + K+(aq) + Cl-(aq) AgCl (s) + K+(aq) + NO3-(aq) Writing Net Ionic Equations 1. Write a balanced molecular equation. 2. Dissociate all strong electrolytes. Write the complete ionic equation. 3. Cross out anything that remains unchanged from the left side to the right side of the equation. 4. Write the net ionic equation with the species that remain. Write the balanced molecular equation, complete ionic equation and net ionic equation for the following: a) FeCl2 (aq) + K2S (aq) b) AlBr3 (aq) + NaOH (aq) c) (NH4)3PO4 (aq) + Ca(NO3)2 (aq) d) Aqueous solutions of silver nitrate and sodium carbonate react. e) Aqueous solutions of barium chloride and potassium sulfate react. • Complete questions 4.22 and 4.24. In each reaction with a precipitate write the balanced molecular equation, complete ionic equation and net ionic equation • Hand in on: Acids: • 2 definitions: »Substances that increase the concentration of H1+ when dissolved in water (Arrhenius). »Proton donors (Brønsted– Lowry). »If an acid can donate more than one proton it is diprotic or triprotic. Acids Strong acids dissociate completely in water There are only seven strong acids: • • • • • • • Hydrochloric (HCl) Hydrobromic (HBr) Hydroiodic (HI) Nitric (HNO3) Sulfuric (H2SO4) Chloric (HClO3) Perchloric (HClO4) Bases: • Substances that increase the concentration of OH1- when dissolved in water (Arrhenius). • Proton acceptors (Brønsted–Lowry). Bases Strong bases dissociate completely in water. The strong bases are the soluble salts of hydroxide ion: • Alkali metals (LiOH, NaOH, etc) • Ca(OH)2, Sr(OH)2, Ba(OH)2 Weak Acids and Bases • are weak electrolytes. • Therefore, they are partially ionized in solution. • HF(aq) is a weak acid; most acids are weak acids. • We write the ionization of HF as: – HF H1+ + F1- Identifying Strong & Weak Electrolytes • Compounds can be classified as by looking at their solubility: • Strong electrolytes: – Ionic compounds. – Strong acids are strong electrolytes. • Weak electrolytes: – Weak acids and bases. • Nonelectrolytes: – All other compounds. • Complete questions 4.35 to 4.38 Acid-Base Reactions In an acid-base reaction, the acid donates a proton (H+) to the base. Neutralization Reactions When solutions of an acid and a base are combined, the products are a salt and water. Molecular Equation: HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) acid base salt water a salt is any non-acidic or basic ionic compound Neutralization Reactions When a strong acid reacts with a strong base, the complete ionic equation is… HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) H+ (aq) + Cl- (aq) + Na+ (aq) + OH-(aq) Na+ (aq) + Cl- (aq) + H2O (l) Neutralization Reactions When a strong acid reacts with a strong base, the net ionic equation is… HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) H+ (aq) + Cl- (aq) + Na+ (aq) + OH-(aq) Na+ (aq) + Cl- (aq) + H2O (l) H+ (aq) + OH- (aq) H2O (l) Mg(OH)2 (s) + HCl (aq) • Molecular Equation: • Mg(OH)2 (s) + 2 HCl (aq) MgCl2 (aq) + 2 H2O (l) • Complete Ionic Equation: • Mg(OH)2 (s) + 2 H1+(aq) + 2 Cl1-(aq) Mg2+(aq) + 2 Cl1-(aq) + 2 H2O (l) • Net Ionic Equation: • Mg(OH)2 (s) + 2 H1+(aq) Mg2+(aq) + 2 H2O (l) • Complete questions 4.39 & 4.40 Gas-Forming Reactions • Reaction of sulfides with acid gives rise to H2S(g). • FeS (s) + 2 HCl (aq) FeCl2 (aq) + H2S (g) • Reaction of sulfites with acid gives rise to SO2 (g). • SrSO3 (s) + 2 HI (aq) SrI2 (aq) + SO2 (g) + H2O (l) Gas Forming Reactions • Acid with carbonate or hydrogen carbonate forms CO2: – CaCO3 (s) + HCl (aq) CaCl2 (aq) + CO2 (g) + H2O (l) – NaHCO3 (aq) + HBr (aq) NaBr (aq) + CO2 (g) + H2O (l) • Bases with ammonium compounds gives ammonia gas: – NH4Cl (aq) + NaOH (aq) NH3 (g)+ H2O (l) + NaCl (aq) • Complete question 4.43 Oxidation-Reduction Reactions • Involve the transfer of electrons • An oxidation occurs when an atom or ion loses electrons. (LEO) • A reduction occurs when an atom or ion gains electrons. (GER) • In all redox reactions, one species is reduced at the same time as another is oxidized. Oxidation Numbers • used to determine if an oxidationreduction reaction has occurred • we assign a charge to each element in a neutral compound or charged entity. • these charges, real or virtual, are called oxidation numbers Rules of Oxidation Numbers • Elements have an oxidation number of 0. • The oxidation number of a monatomic ion is the same as its charge. • Oxygen has an oxidation number of 2- (except peroxides, O22-) • Hydrogen has an oxidation number of 1+ (except hydrides, H1-) • The sum of the oxidation numbers in a neutral compound is 0. • The sum of the oxidation numbers in a polyatomic ion is the charge on the ion. Calculation of Oxidation Numbers • H2O - H is 1+, O is 2• H2O2 – H is 1+, O is 1• AlH3 – Al is 3+, H is 1• CaCl2 – Ca is 2+, Cl is 1- • PbS2 – Pb is 4+, S is 2• Mn3N7 – Mn is 7+, N is 3• H2SO4 – H is 1+, S is 6+, O is 2• Cr2O72– Cr is 6+, O is 2- • complete questions 4.49 and 4.50 Recognizing Oxidation and Reduction • Consider the following balanced chemical equation: • 3 Cu2+ + 2 Al 3 Cu + 2 Al3+ • Let’s apply what we know about oxidation numbers to this equation: • • O.N. 3 Cu2+ + 2 Al 3 Cu + 2 Al3+ 2+ 0 0 3+ • copper goes from 2+ to 0; – it’s charge got lower, so it is reduced (it gained electrons). • The aluminum goes from 0 to 3+; – it’s charge got greater, so it is oxidized (it lost electrons): • • • reduction (gained 2 electrons each) ┌─────────────┐ 3 Cu2+ + 2 Al 3 Cu + 2 Al3+ • O.N. 2+ 0 0 3+ • └──────────────┘ • oxidation (lost 3 electrons each) • The total number of electrons lost and gained is the same; 6 • A redox equation is balanced if: – it is balanced for atoms on each side. – the total electrons lost and gained are equal. • A bit of terminology; – the substance that is oxidized is the reducing agent. – The substance that is reduced is the oxidizing agent. Our equation now looks like this: • • • • • • O.N. • • • oxidizing agent reduction (gained 2 electrons each) ┌───────────┐ 3 Cu2+ + 2 Al 3 Cu + 2 Al3+ 2+ 0 0 3+ └────────────┘ oxidation (lost 3 electrons each) reducing agent Examples: 1. CH4 (g) + 2 O2 (g) CO2 (g) + 2 H2O (l) 2. 2 PbS(s) + 3 O2 (g) 2 PbO (s) + 2 SO2 (g) • Complete questions 4.51 and 4.52 Displacement Reactions • Also called single replacement reactions. • In displacement reactions, one metal replaces another metal in an ionic compound or acids. • The general pattern is: – A + BX AX + B – where • A is a neutral metal • B is a metal cation • X is any anion Example: • metals produce hydrogen gas with acids. • Consider Mg and HCl: – Mg(s) + 2 HCl(aq) MgCl2 (aq) + H2 (g) • the metal is oxidized • H1+ is reduced. Example: • metals can be oxidized in the presence of a salt: – Fe(s) + Ni(NO3)2 (aq) Fe(NO3)2 (aq) + Ni(s) • The net ionic equation shows the redox chemistry well: – Fe(s) + Ni2+(aq) Fe2+(aq) + Ni(s) • iron has been oxidized to Fe2+ • Ni2+ has been reduced to Ni. • Complete questions 4.53 and 4.54 Displacement Reactions In this reaction, silver ions oxidize copper metal. Cu (s) + 2 Ag+ (aq) Cu2+ (aq) + 2 Ag (s) Displacement Reactions The reverse reaction, however, does not occur. Cu2+ (aq) + 2 Ag (s) x Cu (s) + 2 Ag+ (aq) Why? Activity Series • is a list of metals in order of decreasing ease of oxidation. • allows us to predict whether a given displacement reaction will occur. Activity Series • The metals at the top are active metals. • The metals at the bottom are noble metals. • A metal in the activity series can only be oxidized by a metal ion below it. • For example: – If Cu + Ag1+ ions: – Cu2+ ions are formed because Cu is above Ag in the activity series: – Cu(s) + 2 AgNO3 (aq) Cu(NO3)2 (aq) + 2 Ag(s) • or – Cu(s) + 2 Ag1+(aq) Cu2+(aq) + 2 Ag(s) • Complete questions 4.56 to 4.58 Molar Concentration • indicates the amount of solute dissolved in a given quantity of solvent or solution. • is the most common measure of concentration used in the lab. • is the number of moles of solute, per litre of solution (mol/L, mol∙L-1). • is also called Molarity (M). • for instance, 0.12 mol/L ≡ 0.12 M Concentration of an Electrolyte • the concentration of the ions may be different than the calculated concentration of the substance in solution: • If the aluminum sulfate is a 0.200 mol/L solution, then Al2(SO4)3 (s) → 2 Al3+(aq) + 3 SO42-(aq) • 0.200 mol/L 2(0.200 mol/L) 3(0.200 mol/L) • = 0.400 mol/L = 0.600 mol/L • Write the equation for the dissociation of the following ionic substances. Calculate the concentration of each ion in solution: – 0.35 mol/L NaOH – 1.12 mol/L (NH4)2CO3 – 0.056 mol/L V3(PO4)5 Calculating Molarity • Molar concentration (molarity) calculations consist of 3 variables: – concentration (mol/L) – number of moles (mol) – volume (L) • If we know any two of these, we can calculate the third. • Dimensional analysis is very helpful in these calculations. Examples of Molarity • Calculate the molar concentration when 0.256 mol of CuSO4 is dissolved in 1.1 L of solution. • molar concentration = • • c = = = moles of solute volume of solution n v 0.256 mol 1.1 L 0.23 mol/L (2 s.d.) Calculate the molar concentration when 28.2 g of KNO3 is dissolved in 750. mL of solution. • given 28.2 g ; need to convert mass to moles • molar mass: KNO3 • 1xK= 1 x 39.10 g/mol • 1 x N= 1 x 14.01 g/mol • 3 x O= 3 x 16.00 g/mol • 101.11 g/mol • • • moles of substance = = 28.2 g 101.11 g/mol 0.279 mol • • • • • • • • • • • volume must be reported in L: 750. mL x 1L 1000 mL = 0.750 L molar concentration: c = n V = = 0.372 mol/L 0.279 mol 0.750 L Calculate the volume of solution made with 0.455 mol of sodium phosphate in a 2.00 M solution. • c = n V • V = n c • = = 0.455 mol 2.00 mol/L 0.228 L How many moles are present in 4.50 L of a 0.025 mol/L solution of magnesium nitrate? •c = •n = n V cV = (0.025 mol/L)(4.50 L) = 0.11 mol • Complete questions 4.61 to 4.69 Other Measures of Concentration • based on weight to weight (gram to gram) concentration • Parts per hundred (percent, %) – 1 g of a solute per 100 grams of solution. – One part in 102. – equal to 1 g of a solute in 100 g of solution, or 100 mL of solution. Parts per ..... • Parts per thousand – one gram for every 1000 g of solution. – equivalent to: • one drop of ink in a cup of water • one second per 17 minutes. – "Parts per thousand" is often used to record the salinity of seawater. Parts per ... • Parts per million (ppm) – one gram of solute for every 1 000 000 g of solution (1 mg/kg, 1 μg/g) – equivalent to: • one drop of ink in a 150 litre (40 gallon) drum of water • 1 L of ink in Riversdale pool (assume 106 L) • 1 s in 11.57 days – Used to measure levels of metals, nutrients and toxic substances in the environment Parts per ... • Parts per billion (ppb) – one gram of solute for every 109 grams of solution (1 ng/g, 1 μg/kg) – equivalent to: • 1 gram of ink in Riversdale pool. • 1 second in 31.71 years – is the level of activity of hormones in living system. – some carcinogens and toxins are active at this level. Parts per ... Parts per trillion – "ppt” – one gram of solute for every 1012 grams of solution (1 pg/g, 1 ng/kg). – equivalent to: • 1 gram of ink in Blackstrap Lake. • 1 second in 31 700 years Parts per.... Parts per quadrillion (ppq) – one gram of solute for every 1015 grams of solution (1 fg/g, 1 pg/kg). – equivalent to: • 1 gram of ink in Lake Ontario. • 1 second in 31 million years – Very few analytical techniques can measure with this degree of accuracy – it is still used in some mathematical models of toxicology and epidemiology. Dilution Dilution • The stock solution is a more concentrated form of solution (hydrochloric acid is 12.4 mol/L). • use the chemicals in the lab in diluted form. • need to decide how much of the stock solution needed to make the solution you want. Dilution • Have: 12.4 mol/L stock solution HCl • Want: 2.00 L of 0.100 mol/L solution. • First: Calculate the number of moles in the dilute solution: • • • n = cv = (0.100 mol/L)(2.00 L) = 0.200 mol Dilution • Second: Calculate volume of stock solution needed: • • • • v = n c = 0.200 mol 12.4 mol/L = 0.0161 L (1000 mL/L) = 16.1 mL HCl • 16.1 mL HCl stock solution is added to water to make 2.00 L of a 0.100 mol/L solution. Dilution • There is an easier way to do this: • Moles taken from the stock solution (ns) equals the number of moles that goes into the dilute solution (nd) • • and • • ns = nd ns = csvs nd = cdvd • so the formula for dilution is: • csvs = cdvd Dilution • What volume of concentrated sulfuric acid (18 M) is needed to make 500. mL of 2.00 M dilute solution? • CsVs = CdVd • (18 mol/L)Vd = (2.00 mol/L)(500. mL) • Vd = 56 mL concentrated H2SO4 is needed. Dilution • What is the concentration of dilute solution if 25.0 mL of glacial acetic acid (24.0 mol/L) is added to make 2.00 L of dilute solution? • CsVs = CdVd • (24.0 mol/L)(25.0 mL) = (Cd)(2.00 L)(1000 mL/1 L) • Cd = 0.300 mol/L acetic acid. Dilution • What volume of a 1.00 mol/L stock solution of aluminum chloride is needed to make 500. mL of 3.0 x 10-4 mol/L solution? • CsVs = CdVd • (1.00 mol/L)Vd = (3.0 x 10-4 mol/L)(500. mL) • Vd = 0.15 mL AlCl3 stock solution is needed. • Complete questions 4.71 to 4.74 Mixing a Solution Mixing Solutions • Consider these solutions: • • • • • • • CaCl2 (aq) → 0.25 mol/L Mg3(PO4)2 (aq) 0.011 mol/L Ca2+(aq) + 2 Cl1-(aq) 1(0.25 mol/L) 2(0.25 mol/L) = 0.25 mol/L = 0.50 mol/L → 3 Mg2+(aq) + 3(0.011 mol/L) = 0.033 mol/L 2 PO43-(aq) 2(0.011 mol/L) = 0.022 mol/L Mixing Solutions • take 300. mL of the CaCl2 and 200. mL of the Mg3(PO4)2 and we mix them. • Assuming that no precipitate is formed, what will the concentration of each ion in solution be after mixing ? Mixing Solutions • we use the dilution formula: CsVs = CdVd • Cs and Vs is the original concentration and volume of each ion – 0.25 mol/L and 300. mL for the Ca2+ • Vd is the total volume of the mixture – 300. mL + 200. mL. • We are looking for Cd, the ion concentration after mixing: Mixing Solutions • Cd = CsVs Vd • For the Ca2+ ion the calculation is as follows: • [Ca2+] = (0.25mol/L)(300.mL) (300. mL + 200. mL) • = 0.15 mol/L Mixing Solutions • [Cl1-] = • (0.50 mol/L)(300. mL) (300. mL + 200. mL) = 0.30 mol/L • [Mg2+] = • (0.033 mol/L)(200. mL) (300. mL + 200. mL) = 0.013 mol/L • [PO43-] = (0.022 mol/L)(200. mL) (300. mL + 200. mL) • = 0.0088 mol/L • Complete question 4.70 Using Molarities in Stoichiometric Calculations • Consider this problem: – 150. mL of 0.500 mol/L lead (II) nitrate is added to a solution containing sufficient potassium iodide. What mass of lead (II) iodide will precipitate ? Assume the lead (II) iodide is completely insoluble. Step 1 • Write and balance the chemical equation: • Pb(NO3)2 (aq) + 2 KI(aq) PbI2 (s) + 2 KNO3 (aq) Step 2 • List information: • Pb(NO3)2 (aq) + 2 KI(aq) PbI2 (s) + 2 KNO3 (aq) 150. mL g? 0.500 mol/L Step 3 • Convert given information to moles: • Pb(NO3)2 (aq) + 2 KI(aq) PbI2 (s) + 2 KNO3 (aq) 150. mL g? 0.500 mol/L • (0.500 mol/L) (150. mL)(1 L / 1000 mL) = 0.075 mol Step 4 • Use mole ratio to find moles of unknown: • 0.0750 mol Pb(NO3)2 x 1 mol PbI2 1 mol Pb(NO3)2 = 0.0750 mol PbI2 Step 5 • Convert answer to desired units • 0.0750 mol Pb(NO3)2 x 1 mol PbI2 1 mol Pb(NO3)2 = 0.0750 mol PbI2 • 0.0750 mol PbI2 x (207.20 + 2(126.90)) g/mol = 34.6 g PbI2 • Aqueous cadmium chloride reacts with sodium sulfide to produce bright-yellow cadmium sulfide. What mass of cadmium sulfide would be produced if 50.00 mL of a 3.91 M solution of sodium sulfide reacts with sufficient cadmium chloride? • Solutions of calcium nitrate and sodium phosphate react. If 256 mL of a 0.356 M solution of calcium nitrate reacts with sufficient sodium phosphate, what mass of precipitate would result? Titration • The analytical technique in which one can calculate the concentration of a solute in a solution. • is most often done in connection with acid – base reactions. • one substance, of unknown concentration and known volume is placed in a flask. • the other substance of known concentration is placed in a burette. This allows the volume to be read easily. Titration • We are dealing with substances in solution. • The moles of base is equal to: nb = cbVb • and the moles of acid in solution is equal to: na = caVa • Since at the point of neutralization the moles of acid and base are equal, the following formula is arrived at: caVa = cbVb caVa = cbVb • What is the concentration of HCl if 50.00 mL of HCl solution is neutralized by 16.15 mL of 0.100 M NaOH ? – Ca = ? – Va = 50.00 mL – Cb = 0.100 mol/L – Vb = 16.15 mL Ca = (0.100 mol/L)(16.15 mL) 50.00 mL = 0.0323 M NaOH caVa = cbVb • What volume of 0.250 mol/L KOH will be neutralized by 160. mL of 0.400 mol/L HNO3 ? – Ca = 0.400 mol/L – Va = 160. mL – Cb = 0.250 mol/L – Vb = ? Vb = (0.400 mol/L)(160. mL) 0.250 mol/L = 256 mL KOH Indicators • chemicals which are pH sensitive. • undergo a colour change as the pH shifts from acid to basic conditions. • The neutralization point is detected when the solution changes colour. • Indicators are the method of choice in student labs. They are also used in the field, where instrumentation is limited. • Indicators are used in acid-base reactions and a broad range of chemical reactions, including redox reactions Titration • Complete questions 4.77 to 4.86