Unit 7: Enzymes
advertisement

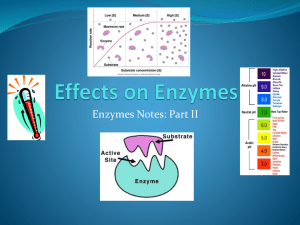
Enzymes are catalysts that regulate the rate of a chemical reactions within the cells. Enzymes lower the activation energy needed to initiate reactions. Enzymes exhibit specificity because of it’s ordered structure. Most enzymes react within an optimum temperature, pH range, and may need the presence of a cofactor. 2 Is a condition that prevents the metabolism of lactose due to the lack of the enzyme responsible for it. Lactose is a disaccharide composed of β-D-galactose and β- D-glucose. Lactase is the enzyme needed to brake down lactose. It is found in the small intestine, liver and kidney of mammals. Lactase breaks down lactose to its components β-D- galactose and β-D-glucose which can be used as a source of energy by the cells. 3 Reactions catalyzed by enzymes have a temperature range in which the reaction will occur at an optimum level. Within this range, the reaction rate increases 10% per every 1°C increase in temperature. Temperature well above or below this range can change the structure of the enzyme. When the structure of the enzyme changes, it is denatured and will no longer act as a catalyst. We will observe the effects of different temperatures on the enzyme activity. Temperatures: 0-5, 20-25, 40, 60, 80, 100°C 4 25° 0-5° 3 water baths each with a specific temperature 100° 5 Substrate and enzyme are put in a microfuge tube, and placed in the proper water bath for 10 minutes. Microfuge tube Substrate Enzyme Sample in water bath 6 Glucose strip is used to measure the amount of glucose present in each sample. mg/mL of glucose 7 The results are as following: T (°C) 0 25 40 60 80 100 Glucose mg/mL 0 600 1000 800 300 0 Using these results a graph is plotted 8 mg/mL Glucose Temperature (°C) The conclusion is that 40°C is the temperature where the enzyme works best. 9 pH can also affect the activity of an enzyme. Too high or too low pH may hamper the enzyme’s activity by denaturing it. There is an optimal pH, which is that where the enzyme efficiency is at its best. Remember this enzyme is found in the small intestine, liver and kidney, and the pH there tends to be slightly basic. 10 7 different buffers ranging from 2 to 12 are used in this experiment. Samples (buffer, milk and enzyme) are kept at a constant temperature of 40°C for 10 minutes and then tested with the glucose strip. 11 The results areas following Using the results a graph was plotted 12 1200 1000 800 600 Glucose 400 200 0 -200 0 5 10 15 pH The conclusion is pH 8 is where this enzyme works best. 13 Enzymes exhibit specificity. They are globular in shape and have one or more active sites each of which is structured so that it bonds with a specific substrate. 14 The specificity of the enzyme is tested on two substrates, maltose and lactose (found in milk). 15 2 microfuges are filled each with one of the substrates plus the enzyme and put in the water bath of 40°C for 10 minutes. The amount of glucose is then tested with the glucose strip. Results 16 Cofactors are inorganic compounds, usually metallic ions, that are a part of the active site of enzymes. Cofactors make the formation of an enzyme-substrate complex possible. Copper and magnesium are the cofactors for lactase. EDTA binds copper and makes it unavailable for use as a cofactor. 17 Two microfuge tubes (control and EDTA) milk is put in, to the control dH2O is added, to the other EDTA is added. Both tubes then receive the enzyme and are placed in the 40°c water bath for 10 minutes. Both tubes are then tested with the glucose strip. Results The End 18