Powerpoint
advertisement

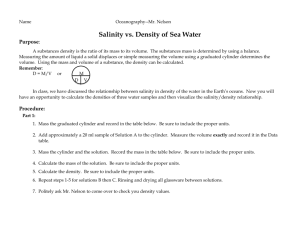
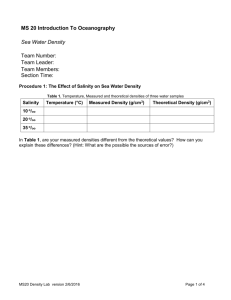
Chapter 5 Water and Seawater Essentials of Oceanography 7th Edition Atomic structure Atoms are the building blocks of all matter Nucleus contains: Neutrons (no charge) Protons (+ charge) Outer shell(s) contain: Electrons (– charge) Figure 5-1 The water molecule Composed of 1 oxygen and 2 hydrogen atoms (H20) Contains strong (covalent) bonds between atoms Unusual bend in geometry Has polarity (oppositely charged ends) Figure 5-2a Interconnections of water molecules Polarity causes water molecules to form weak (hydrogen) bonds between water molecules Water sticks to itself and to other substances Allows water to be the universal solvent Figure 5-3 Water as a solvent Water dissolves table salt (NaCl) by attracting oppositely charged particles Pulls particles out of NaCl structure to dissolve it Figure 5-4 Water in the 3 states of matter Latent (hidden) heat = energy that is either absorbed or released as water changes state Figure 5-5 The ocean moderates coastal temperatures Water has high heat capacity, so it can absorb (or release) large quantities of heat without changing temperature Moderates coastal temperatures Figure 5-6 Hydrogen bonds in H2O Figure 5-8 The formation of ice As water cools to 4°C: Molecules slow Water contracts Density increases Below 4°C: Hydrogen bonds form Water expands As water freezes: Expands by 9% Figure 5-11 Snowflake geometry All snowflakes have 6-sided geometry Caused by water’s polarity and ability to form hydrogen bonds Figure 5-12 Salinity Salinity = total amount of solid material dissolved in water Can be determined by measuring water conductivity Typically expressed in parts per thousand (‰) Figure 5-15 Constituents of ocean salinity Average seawater salinity = 35‰ Main constituents of ocean salinity: Chloride (Cl–) Sodium (Na+) Sulfate (SO42–) Magnesium (Mg2+) Figure 5-13 Salinity variations Location/type Salinity Normal open ocean Baltic Sea Red Sea Great Salt Lake Dead Sea Tap water Premium bottled water 33-38‰ 10‰ (brackish) 42‰ (hypersaline) 280‰ 330‰ 0.8‰ or less 0.3‰ Ocean buffering Ocean pH = 8.1 (slightly basic) Buffering protects the ocean from experiencing large pH changes Figure 5-18 Processes affecting seawater salinity Processes that decrease seawater salinity: Precipitation Runoff Icebergs melting Sea ice melting Processes that increase seawater salinity: Sea ice forming Evaporation The hydrologic cycle Figure 5-19 Surface salinity variation Pattern of surface salinity: Lowest in high latitudes Highest in the tropics Dips at the Equator Surface processes help explain pattern Figure 5-20 Surface salinity variation High latitudes have low surface salinity High precipitation and runoff Low evaporation Tropics have high surface salinity High evaporation Low precipitation Equator has a dip in surface salinity High precipitation partially offsets high evaporation Global surface salinity Figure 5-21 Salinity variation with depth Curves for high and low latitudes begin at different surface salinities Halocline = layer of rapidly changing salinity At depth, salinity is uniform Figure 5-22 Seawater density Factors affecting seawater density: Temperature ↑, Density ↓ (inverse relationship) Salinity ↑, Density ↑ Pressure ↑, Density ↑ Temperature has the greatest influence on surface seawater density Density and temperature variations with depth Figure 5-24 Pycnocline and thermocline Pycnocline = layer of rapidly changing density Thermocline = layer of rapidly changing temperature Present only in low latitude regions Barrier to vertical mixing of water and migration of marine life Ocean layering based on density Mixed surface layer (surface to 300 meters) Low density; well mixed by waves, currents, tides Upper water (300 to 1000 meters) Intermediate density water containing thermocline, pycnocline, and halocline (if present) Deep water (below 1000 meters) Cold, high density water involved in deep current movement Seawater desalination Distillation Desalination methods: Figure 5-25 Distillation Solar Heat Electrolysis Reverse osmosis Freeze separation Reverse Osmosis Figure 5-26 End of Chapter 5 Essentials of Oceanography 7th Edition