6 Gases - Science
advertisement

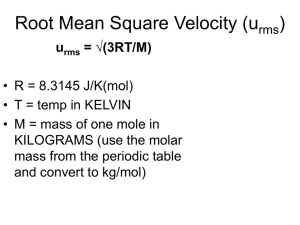
6 Gases A gas expand to occupy the entire volume it is placed in. Molecules in a gas translate freely between collisions, and they all behave alike regardless of their type. What are some of the properties of gases? Pressure, temperature, heat capacity, volume, density, molar volume, color, average speed of molecules, solubility (in water or other liquid), absorption, compressibility, gas-liquid equilibrium, composition, identity (compound or element), chemical properties, combustibility, stability Which of these properties are intensive properties, and which are extensive properties? 6 Gases 1 Announcement Appointments for Winter '04 enrolment are now posted on your QUEST account – Each student has only a three day appointment time. If missed, you will have to wait until all students have completed their appointments. Open enrolment begins November 3rd, but courses could be full by then CHECK YOUR QUEST ACCOUNT FOR YOUR APPOINTMENT as soon as possible. 6 Gases 2 6 Gases 3 Pressure Pressure: force per area (1 Pascal = 1 N m–2) Liquid pressure (explain these formulation in terms of physics) F W g*m g* V*d g * h *A* d P = --- = ---- = ---------- = --------------- = ----------------- = g * h * d A A A A A These equivalences are useful for unit conversions: 1 atm = 101.325 kPa = 76 cm Hg = 760 mm Hg (torr in honor of Torricelli) = 1.01325 b = 1013.25 mb (bar & m bar) = 14.6960 pounds / sq. inch 6 Gases 4 Torricelli’s Barometer Barometric pressure Explain Torricelli’s work (1608 - 1647) P=ghd 1 atm = 0.76 m Hg 13594 kg 1 m3 Hg 9.80665 N 1 kg = 101317 N m–2 (Pascal) = 101.32 k Pa 6 Gases 5 Torricelli Mercury Barometer Evangelista Torricelli invented the Torricelli Mercury Barometer in 1644. He used a long glass tube, closed at the upper end, open at the lower and filled with mercury. 6 Gases 6 No water!! 1 Pump Water from a Well 0 The specific gravity of mercury is 13.5939. If water is used for a . barometer, what is the height of water corresponding to 1.00 atm? 3 Solution: 3 13.5939 g 1 cm3 H2O = 1033.14 cm H O 76 cm Hg 2 1 cm3 Hg 1g = 10.33 m H2O m W a t e r Explain water pump and depth of well Water water everywhere! 6 Gases What about a diver under water? Be sure to get that during lecture. 7 Pressure of Skater A skater weighing 70 kg stands on one foot and the contact between the blade and ice is 1 cm2. What is the pressure in torr sustained by the ice? Solution: 70 kg 1 cm2 10000 cm2 1 m2 1 m3 Hg 13594 kg 1000 mm = 51493 mm Hg 1m = 51493 torr = 67.8 atm 6 Gases 8 Avogadro’s Law The ABCD of gas laws are Avogadro’s, Boyle’s, Charle’s & Dalton’s laws of gases. Avogadro’s hypothesis: proposed in 1811 at the same temperature T and pressure P, equal volumes contain equal amounts of gases in moles n. at the same temperature T and pressure P, the volume V of a gas is proportional to the number of molecules or number of moles n. VP,T = k n (k, a constant, 22.4 L at STP) Explain Avogadro’s hypothesis and implications Explain Avogadro’s law in your language 6 Gases Avogadro’s scientific contributions to science will be given. 9 Boyle’s Law Robert Boyle, (1621-91) For a certain amount (constant n) of gas at constant temperature T, the volume V times the pressure P is a constant. Mathematical aspects of PV product will be discussed P P V n, T = constant = P1 V1 = P2 V2 • P1 V1 How do you graph the Boyles law? State the law in another way. T2 n1 T1 n1 • P2 V2 V What curves are P-V plots? 6 Gases 10 Charle’s Law (law of Charles-Gay-Lussac) For a certain amount of gas at constant pressure, its volume, V, is directly proportional to its temperature T in Kelvin. Vn, P = b T or Pn, V = b T (b is a constant) (b is a constant) Vn, P or Pn, V State the Charle’s law in another way V1 V2 ----- = ----- = b, V1T2 = V2 T1 T1 T2 Charles, Jacques-Alexandre-César (1746-1823, top) first to ascend in a H2-balloon, developed this law in 1787. Later Joseph Louis Gay-Lussac 6 Gases (lower) published a paper citing Charles’s law. T 11 General Gas Equation Combining ABC gas laws, we have P1 V1 T1 = P2 V2 T2 =nR Subscripts 1 and 2 refer to different conditions for the same quantities of gases (n). Experiments show that one mole of gas at STP occupies 22.4 L. 6 Gases 12 The Ideal Gas Equation The ABC laws of gases can be combined into one and the result is an ideal gas equation. A+B+C ideal PV=nRT 1 atm * 22.4140 L R = ---------------------------- = 0.082057 L atm mol–1 K–1 1 mol 273.15 K 101.325 kPa * 22.414 L R = ----------------------------------- = 8.3145 L kPa mol–1 K–1 1 mol 273.15 K Please confirm that 1 kPa L = 1 J (1 L = 1e-3 m3) 6 Gases 13 Dalton’s law The Partial pressure Pi is the pressure of a component in a mixture as if others don’t exist in that system – due to the fact all gases behave as if they are independent of each other. Pi = ni R T correction V Dalton’s law: The total pressure Ptotal, of a mixture of gases is the sum of the partial pressures of the components. Ptotal = P1 + P2 + … + Pn = (n1 + n2 + … + nn) RT V 6 Gases (V is common to all) 14 Application of the Ideal gas Law Parameters of the ideal gas law: P, V, T, n and a constant R ideal A+B+C PV=nRT At constant n and T, P1 V1 = P2 V2 Ideal gas law Boyles law At constant n and V P= (n R / V ) T P1 / T1 = P2 / T2 Charles law At constant n and P V = (n R / P ) T V1 / T1 = V2 / T2 ditto At constant P and T V = (R T / P ) n V=k n Avogardro’s law 6 Gases 15 Gas Densities Evaluate the density of O2 (molar mass M = 32.0) at 300 K and 2.34 atm. Hint: Density d of a gas with mass m (= n M) and volume V m nM d = ----- = -----V V n d ---- = -----V M Thus nRT dRT P = ------------- = ----------V M Find relationship between density d and M. Manipulate symbols to get a useful formula before you calculate the quantities. d=PM/RT Include and work out the units plse d = 2.34 *32.0 / (0.08205*300) = ___ 6 Gases 16 Reactions Involving Gases How much NaN3 is required to produce 12.0 L N2 gas at 302 K and 1.23 atm for the air bag in your designed Autotie? Solution: Equations: 2 NaN3 = 2 Na + 3 N2; 12.0 L N2 1.23 atm mol K 0.08205 L atm * 302 K n = V (P / R T ) 2 mol NaN3 3 mol N2 (23+3*14) g NaN3 1 mol NaN3 = ___ Work out N2 volume for 51 g NaN3 used under the same condition. 6 Gases 17 - reaction involving gases NO is made from oxidizing NH3 at 1123 K with platinum as a catalyst. How many liter of O2 at 300 K and 1 atm is required for each liter of NO measured also at 300 K and 1 atm? Solution: The reaction is: 4 NH3 + 5 O2 4 NO + 6 H2O Since n = ( P/RT ) V molar relationships are the same as volumetric relationship, providing T and P are the same. Complicated problem may 1 L NO 5 mol O2 = 1.25 L O2 4 mol NO have a simple solution, Further oxidation of NO leads to NO2, which is used to make HNO3, a valuable commodity. How much H2O is produced? 6 Gases 18 A Mixture of Gases What is the pressure exerted by the gas when 1.0 g of H2, 2.0 g of O2, and 0.1 g of CO2 are all confined in a 10.0 L cylinder at 321 K? Solution: Dalton’s law : ntotal = i ni (count molecules non-discriminately) P = n R T / V; 1 mol 1.0 g H + 2.0 g O2 n= 2 2gH 2 P = 0.585 mol 8.314J * 321 K 10 L mol K 1 mol + 0.1 g CO2 32 g O2 = 126 kPa Calculate partial pressures of each gas plse 6 Gases 1 mol 44 g CO2 = 0.585 mol (note 1 J = 1 kPa L) 19 Vapor pressure The (saturated) vapor pressure is the partial pressure that is at equilibrium with another phase. Vapor pressure of ice Vapor pressure of water Explain Structure of water molecule Hydrogen bonding Structure of water Structure of ice Vapor pressure of ice and water Relative and absolute humidity 6 Gases 20 Collecting Gas Over Water When 1.234 g of a sample containing Ag2O is heated, 40.6 mL of O2 is collected over water at 296 K, and the atmosphere is 751 mmHg. Vapor pressure of water at 296 K is 21.1 mmHg. What is the percentage of Ag2O in the sample? Solution: Ag2O = 2 Ag + 0.5 O2 n = P V / R T; R = 0.08205 L atm mol-1 K-1 P = (751 – 21.1) mmHg / 760 mmHg = 0.961 atm (PO2 = Ptotal – Pwater) V = 40.6 mL = 0.0406 L Mass of Ag2O = 0.961 atm*0.0406 L O2 0.08205 L atm mol-1 K-1*296 K 1 mol Ag2O 0.5 mol O2 231.7 g Ag2O 1 mol Ag2O = 0.744 g Ag2O Percentage of Ag2O = 0.744 g Ag2O / 1.234 g = 0.603 Ag2O = 60.3 % Ag2O 6 Gases 21 Assumptions of Kinetic-molecular Theory 1. Gas is composed of tiny, discrete particles (molecules or atoms). 2. Particles are small and far apart in comparison to their own size. 3. Ideal gas particles are dimensionless points occupying zero volume. 4. Particles are in rapid, random, constant straight line motion. 5. There is no attractive force between gas molecules and between molecules and the sides of the container. 6. Molecules collide with one another and the sides of the container. 7. Energy is conserved but transferred in these collisions. 8. Energy is distributed among the molecules in a particular fashion known as the Maxwell-Boltzmann Distribution. 6 Gases 22 Kinetic-molecular Theory of Gases For N gas molecules, molecule mass = m, molecular mass = M speed = u, average speed = u , Avogadro’s number N volume = V, temperature = T, Pressure = P, Kinetic energy = ½ m u 2 Collision frequency u N / V Pressure (m v) (u) (N / V) (N / V) m u 2 = 1/3 (N / V) m u 2 (1/3 due to 3-Dimensional space) P V = 1/3 N m u 2 = n R T (Meaning of T) Thus, 3 R T = NA m u 2 correction Furthermore, u 2 = 3 R T / M (Temperature and speed) 6 Gasesthese formulas for sciences Explain the significances of and apply 23 Molecular Speeds Distributions of speed of various gases will be demonstrated using a simulation program, and for each gas, three speeds are indicated. In the following: m = mass of a molecule, M = molar mass, R = gas constant, and k = R / Navogadro = Boltzmann constant. The most probable speed ump = (2 k T / m)1/2 = (2 R T / M)1/2 The root-mean-square speed urms = ( 3 k T / m)1/2 = (3 RT / M)1/2 The mean or average speed uave = ( 8 k T /π m)1/2 = (8 RT / π M)1/2 6 Gases 24 Diffusion of Gases All gases move together because they are subjected to the same pressure head. Different gases diffuse at different rates Diffusion contributes to net movement of O2 and CO2 across the alveolar-capillary membrane (breathe). Constant molecular motion. Diffusion from higher to lower concentration regions. Since uave = ( 8 k T /π m)1/2 = (8 RT / π M)1/2, (slide 24) 1 Diffusion rate -------Graham’s law M Discuss diffusion rates of H2, He, CH4, N2, O2, CO2, 235UF6, 238UF6 at lecture 6 Gases 25 Diffusion problems Problems are usually to compare diffusion or effusion rates in the following terms: 3RT urms = ( 3 k T / m)1/2 = M uave = ( 8 k T /π m)1/2 = 8RT πM diffusion rate 1 M effusion time for same amount M distance traveled by molecules in certain period amount of gas effused 6 Gases 26 Comparing Effusion Rates If 1e20 N2 molecules effuse from an orifice in 1.0 min, how many H2 molecules will effuse the same orifice at the same condition (T P)? How many minutes will be required for the same number of H2 molecules to effuse? H2 effusion rate = MN2 / MH2 (N2 effusion rate) = (28 / 2 )1e20 molecules/min) = __________ figure out value and units Time for 1e20 H2 molecules to effuse = (2 / 28 ) 1.0 min = __________ What is the effusion rate ratio of N62 Gases and H2 or any two gases? 27 Effusion and Life Breathing chemistry is complicated, and we can only scratches the surface! O2 P2 CO2 A A D Vgas ( P1 P2 ) T P1 T 6 Gases 28 The van der Waals equation for real gases Gases tend to behave ideally at high T and low P. Required T and P for ideality depends on gas properties and molar mass, and van der Waals proposed correction terms for the ideal gas equation for real gases. Correction for intermolecular forces Correction for volume of molecules 2a n (P + ) (V – n b) = n R T 2 V where a and b are gas-dependant constants. Gas He Ne N2 O2 CO2 Cl2 a L2 atm mol-2 b L mol-1 0.0341 0.0237 0.211 0.0171 1.39 0.0391 1.36 0.0318 3.59 0.0427 6.49 0.0562 Explain the meaning of vdW eqn Note units for a and b 6 Gases 29 Application of van der Waal’s Equation What is the pressure of Cl2 at 300 K occupying 20.0 L according to vdW and ideal gas laws? Solution: Look up data for Cl2, a = 6.49 L2 atm mol-2, b = 0.0562 L mol-1, n = 1 mol, R = 0.08205 L atm K-1 mol-1 P= = nRT V–nb - n2a V2 1 mol * 0.08205 L atm mol-1 K-1*300 K 20.0 L – 1 mol 0.0562 mol-1 L 2 mol2 * 6.49 L2 atm mol-2 1 20.02 L2 = _____________________ Please calculate the results, and P from the ideal gas law. 6 Gases 30 Problems related to van der Waal’s Equation What is the molar volume of Cl2 at 300 K and 1 atm according to vdW? Solution: Look up data for Cl2, a = 6.49 L2 atm mol-2, b = 0.0562 L mol-1, n = 1 mol, R = 0.08205 L atm K-1 mol-1 V= nRT + n b = 24.615 / (1+0.013) = 24.299 L 2 2 P+n a/V try V = 22 L = 24.625 / (1+ 0.011) = 24.434 L = 24 L try V = 24.434 L = ? and calculate V Calculate P for a definite volume is easier, and using the successive method for V is interesting, but it’s a challenge. 6 Gases Find out how engineers deal with real gases. 31 Volume of vdW eqn What is the volume of occupied by 132 g CO2 gas at 12.5 atm and 300 K? Solution: Solve volume of van der Waals equation for V 2a 2ab R T + b P n n 2 V –n( )V + ( )V–( )=0 P P P 3 Derive please a = 3.59 L2 atm mol-2, b = 0.0427 L mol-1, n = 1 mol, R = 0.08205 L atm K-1 mol-1 This is similar to problem 6 –106, a question for 6 Gases practicing the successive approximation method. 32 Successive Method again What is the volume of occupied by 132 g CO2 gas at 12.5 atm and 300 K? Solution: a = 3.59 L2 atm mol-2, b = 0.0427 L mol-1, T = 300 K n = 132/44 = 3 mol, R = 0.08205 L atm K-1 mol-1 V= nRT + nb 2 2 P+n a/V = 3*0.08205*300 / (12.5 + 32* 3.59 / 12) + 3*.0427 = 1.78 L = 73.845 / (12.5 + 32*3.59 / 32) + 0.128 = 4.7 L = 73.845 / (12.5 + 32*3.59 / 42) + 0.128 = 5.2 L = 73.845 / (12.5 + 32*3.59 / 52) + 0.128 = 5.5 L = 73.845 / (12.5 + 32*3.59 / 5.62) + 0.128 = 5.58 L 6 Gases 33 Molecular Formula of Gas Combustion of 1.110 g hydrocarbon produces 3.613 CO2 and 1.109 g H2O. A 0.288 g sample of the same has a volume of 131 mL at 298 K and 753 mmHg. Find the molecular formula. Solution: C : H = 3.613 : 1.109*2 = 0.0821 : 0.123 = 1 : 1.5 = 2 : 3 44 18 Empirical formula is C2H3 dRT –1) * 0.08205 L atm mol–1 K –1 * 298 K (0.288 g / 0.131 L M= P = (753 / 760) atm = 54.3_______ work out units C4H6 has a molar mass of 54.3. Confirm and conclusion please! 6 Gases A 2-step problem, similar to one in Advanced Exercises 34 ROOMS FOR TEST #1 (CHEM 120) – Wed., Oct. 8th Write the test during your regular lecture time (10:30 am) on Wed., Oct. 8th. Go to DC 1350 and ESC 146/149 according to your Surnames Surnames For 8:30 class For 9:30 class A–L M–Z A – Mo Mu – Z Room(s) DC 1350 ESC 146 & 149 DC 1350 ESC 146 & 149 10:30 am A – Ma DC 1350 (For you) Mc – Z ESC 146 & 149 (First Year Chem Lab) For 11:30 class A – Ma Mc – Z DC 1350 ESC 146 & 149 6 Gases 35 Regarding Test 1 Do not enter the room until directed to do so by the proctors. We need space and time to set out the test booklets and computer answer cards. Bring a calculator and a pencil for filling out the computer answer card. Do NOT bring your own scrap paper or periodic table. All work must be done on the test booklet. A periodic table will be supplied. 6 Gases 36 Some concepts to review Convert between mass and mole and vice versa Find empirical and molecular formulas Figure out limiting and excess reagent, calculate theoretical and percent yields Calculate concentrations in molarity, mass percentage, etc even when solutions are combined (dilution) Analyze binary mixture: extra problems B2 and B3 (handout page 8) Figure out the net ionic reaction equations Balance redox reaction equations (figure out oxidation states, balance half-reaction equations and balance equations) 6 Gases 37 Concepts to review – cont. Apply ideal gas low to various problems Calculate stoichiometric quantities using on gas law and reaction equations. Apply Dalton’s partial pressure equation Compare effusion or diffusion rates of gases 6 Gases 38