OR IN SIEVE CELLS
advertisement

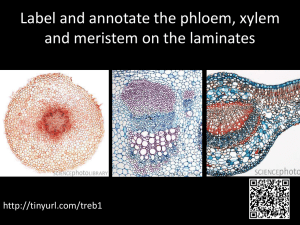
PHLOEM TRANSLOCATION 1. THE EVOLUTION OF AERIAL SHOOTS AND SUBTERRANEAN ROOTS NECESSITATED A MECHANISM FOR LONG-DISTANCE TRANSPORT OF SUGARS. 2. THE PRIMARY FUNCTION OF THE PHLOEM IS TO CARRY OUT THE LONG DISTANCE TRANSLOCATION OF SUGARS AND OTHER PHOTOSYNTHETIC PRODUCTS. 3. PHLOEM TRANSLOCATION OCCURS IN EITHER SIEVE TUBE ELEMENTS STACKED INTO SIEVE TUBES (ANGIOSPERMS), OR IN SIEVE CELLS (GYMNOSPERMS). 1o PHLOEM 1o XYLEM VASCULAR BUNDLE Cork 2o PHLOEM VASCULAR CAMBIUM 2o XYLEM P-PROTEIN (aka: Slime) 1. P-protein found in all dicots, many monocots. It is absent in gymnosperms. 2. Occurs in different forms: tubular, fibrillar, granular, and crystalline. 3. Begin as discrete spherical bodies (P-protein bodies) which gradually disperse during maturation of sieve tube member. 4. Function in sealing off damaged sieve tube elements by plugging up the sieve plate pores. 5. In Cucurbita it consists to two major proteins: PP1 (the filament protein) and PP2 (a lectin, or sugar-binding protein). 6. Callose (-1,3-glucan) used for long term plugging of damaged or senescing sieve tube elements. Companion cell Sieve tube elements Parenchyma cell Unobstructed sieve plate pores Sieve element Parenchyma cell Sieve plate Companion cell Sieve cell P Sieve cell SER Sieve area P THREE TYPES OF COMPANION CELLS IN THE MINOR VEINS OF MATURE EXPORTING LEAVES 1. ORDINARY COMPANION CELLS - Have chloroplasts and a smooth cell wall; relatively few plasmodesmata connect ordinary companion cell to cells other than adjacent sieve element; symplastically isolated. 2. TRANSFER CELLS - Like ordinary companion cells except have finger-like wall ingrowths which increase surface area. Both ordinary companion cells and transfer cells are symplastically isolated and are therefore specialized for taking up sugars from the apoplast. 3. INTERMEDIARY CELLS - Connected to surrounding cells via numerous plasmodesmata and are thus suited for taking up solutes via the symplast; lack well-developed chloroplasts. Sieve Elements Intermediary Cell Ordinary Companion Cell Sieve element Wall ingrowths Transfer cell Plasmodesmata Parenchyma cell Vascular parenchyma cell Intermediary cell plasmodesmata Sieve elements Bundle sheath cells PATTERNS OF TRANSLOCATION: SOURCE TO SINK 1. Phloem sap is not translocated exclusively in either an upward or downward direction and is not influenced by gravity; phloem translocation occurs from source to sink. 2. Sources include any exporting organ, typically mature leaves exporting photosynthate. 3. Storage organs (roots, tubers, seeds, etc.) can also serve as sources. 4. Sinks include any nonphotosynthetic organ or tissue that does not produce sufficient photosynthate to support its own metabolic needs: roots, underground stems, buds, immature leaves, flowers, fruits, etc. SOURCE TO SINK PATHWAYS FOLLOW ANATOMICAL AND DEVELOPMENTAL PATTERNS 1. Proximity is important: upper mature leaves supply photosynthate to growing shoot tip; lower leaves supply the root; middle leaves supply both. 2. Development influences transport: a. Young leaves begin as sinks, gradually become sources. b. Reproductive structures become dominant sinks during flowering. 3. Vascular connections important; source leaves preferentially supply sinks to which they have vascular connections; typically, sources leaves supply sinks along the same vertical row or orthostichy. 4. Phloem interconnections (anastomoses) can provide alternative pathways in the event of wounding or pruning. CHANGES IN SOURCE-SINK RELATIONS DURING LEAF DEVELOPMENT Mature Leaf Young leaf SINK SOURCE TRANSITION FROM SINK TO SOURCE LEAF: AUTORADIOGRAPHIC EVIDENCE USING SUMMER SQUASH (Cucurbita pepo) APHIDS “Honey dew” “stylet” Phloem Sieve Tubes RATES OF PHLOEM TRANSPORT 1. The rate of phloem transport can be expressed as the linear velocity (m/hr) or as the mass transfer rate (g/hr/cm2). 2. A typical velocity of transport is 0.3 - 1.5 m/hr, much faster than the rate of diffusion. 3. A typical mass transfer rate is 1-15 g/hr/cm2. (See Web Topic 10.4 for methods for measuring the mass transfer rates.) 4. Aphids can be used to study transport rates as well as the composition of phloem sap. THE PRESSURE-FLOW MODEL OF PHLOEM TRANSLOCATION 1. ACCORDING TO THE MÜNCH PRESSURE FLOW MODEL, SUGARS MOVE BY BULK FLOW IN SIEVE TUBES IN RESPONSE TO AN OSMOTICALLY GENERATED PRESSURE GRADIENT (p) BETWEEN THE SOURCE AND THE SINK. TRANSLOCATION IS THUS PASSIVE. 2. ATP-DEPENDENT PHLOEM LOADING OF SUGARS OCCURS AT THE SOURCE; ATP-DEPENDENT PHLOEM UNLOADING OCCURS AT THE SINK. LOADING AND UNLOADING ARE THUS ACTIVE. PREDICTIONS OF THE PRESSURE-FLOW MODEL HAVE BEEN CONFIRMED 1. Sieve plate pores must be unobstructed for pressure-flow to occur between sieve tube members. CONFIRMED BY ELECTRON MICROSCOPY PREDICTIONS OF THE PRESSURE-FLOW MODEL HAVE BEEN CONFIRMED 2. True bidirectional transport in a single sieve tube cannot take place. CONFIRMED BYAPHID STUDIES USING RADIOACTIVE TRACERS AND DYES. PREDICTIONS OF THE PRESSURE-FLOW MODEL HAVE BEEN CONFIRMED 3. PHLOEM TRANSLOCATION SHOULD BE A PASSIVE PROCESS, NOT DIRECTLY DEPENDENT ON ATP. CONFIRMED BY PHYSIOLOGICAL STUDIES USING COLD TREATMENT, ANOXIA, AND INHIBITORS. PREDICTIONS OF THE PRESSURE-FLOW MODEL HAVE BEEN CONFIRMED 4. THERE MUST BE PRESSURE GRADIENTS BETWEEN SOURCE AND SINK SUFFICIENT TO DRIVE BULK FLOW. CONFIRMED BY STUDIES USING APHID STYLETS AS MICROMANOMETERS. Vascular parenchyma cells Sieve elements Companion cells Bundle Sheath Cell TWO PATHWAYS OF PHLOEM LOADING POLYMER-TRAPPING MODEL OF PHLOEM LOADING TYPES OF PHLOEM UNLOADING PATHWAYS