4 - Regulation of the Heartbeat
advertisement

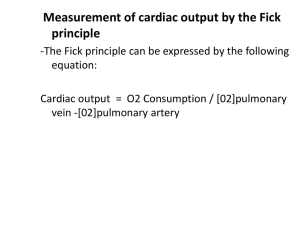
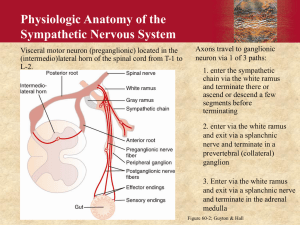
Regulation of the Heartbeat Outline Control of the heart beat Intrinsic control of contractility Extrinsic control of contractility Control of the heartbeat Parasympathetic pathway Parasympathetic fibers originate in the nucleus ambiguus. They pass through the mediastinum to synapse with postganglionic cells on the epicardial surface or within the walls of the heart itself. The right vagus nerve effects the SA node predominantly. The left vagus nerve mainly inhibits AV conduction. Control of the heartbeat Acetylcholine is rapidly hydrolyzed via nodal cholinesterase so the effect is brief. The muscarinic receptors are coupled directly to the acetylcholine-regulated potassium channels by a G protein; this direct coupling allows a prompt response Parasympathetic predominate over sympathetic effects at the SA node. This is mediated by suppressing release of norepinephrine from the sympathetic nerve endings by acetylcholine Control of the heartbeat Sympathetic pathway The cardiac sympathetic fibers originate in the interomedial lateral columns of the T1-5 and C7-8 segments of the spinal cord. Preganglionic and postganglionic neurons synapse mainly in the stellate and middle cervical ganglia. On reaching the base of the heart, these fibers are distributed to the various chambers as an extensive epicardial plexus. Control of the heartbeat The effects of sympathetic stimulation decay very gradually after stimulation. The response to sympathetic activity depends mainly on the intracellular production of second messengers, mainly cAMP Control of the heartbeat Baroreceptor reflex Acute changes in blood pressure elicit inverse changes in heart rate via the baroreceptors located in the aortic arch and carotid sinuses Bainbridge reflex A fluid bolus accelerates the heart rate whether arterial blood pressure rise or not Tachycardia occurs with CVP rises sufficient to distend the right heart. Increases in blood volume not only evoke the Bainbridge reflex, but they also activate the baroreceptor reflex that tend to slow the heart rate. The actual change in heart rate evoked by an alteration of the blood volume is the results of these antagonistic reflex effects. Control of the heartbeat How does the Bainbridge reflex work? Atria have receptors that influence heart rate located in the venoatrial junction. Distention of these receptors send impulses centrally in the vagus nerve. The efferent impulses are carried by fibers from both autonomic divisions to the SA node. The increase in sympathetic activity is restricted to the heart rate; there is no increase of sympathetic activity to the peripheral arterioles nor increase in contractility. Control of the heartbeat Stimulation of atrial receptors also increases urine volume. Atrial natriuretic peptide is released from atrial tissue in response to stretch of the atrial wall. It has potent diuretic and natriuretic effects on the kidneys and dilates blood vessels. Control of the heartbeat Respiratory variation Heart rate accelerates during inspiration and decelerates during expiration. Activity increases in the sympathetic nerve fibers during inspiration, whereas activity in the vagal nerve fibers increases during expiration. Acetylcholine released at the vagal endings is hydrolyzed so rapidly that the rhythmic change in activity are able to elicit rhythmic variations in heart rate. Conversely, norepinephrine is released at the sympathetic endings is removed more slowly, thus dampening out the effects of rhythmic variations in norepinephrine released on heart rate Control of the heartbeat Hence, rhythmic changes in heart rate arise almost entirely from oscillations in vagal activity. During inspiration, venous return to the right side of the heart accelerated and elicits the Bainbridge reflex. After the time delay required for the increased venous return to reach the left side of the heart, left ventricular output increases and raises arterial blood pressure. This reduces heart rate reflexively through the baroreceptor stimulation. Intrinsic control of contractility Frank-Starling mechanism When the load on the heart is increased, it responds with a more forceful contraction. In this experiment the right atrial pressure [preload] was increased. The width of the tracing reflects the stroke volume. For several beats after the rise in preload, the ventricular volume progressively increased. Intrinsic control of contractility During a given systole, the volume of blood expelled was not as great as the volume that had entered. This accumulation of blood dilated the ventricles and lengthen the individual myocardial fibers in the wall of the ventricle. Increased fiber length alters cardiac performance mainly by changing the calcium sensitivity of the myofilaments and, in part, by changing the number of monofilament cross bridges that can interact. Intrinsic control of contractility Increased afterload Changes in diastolic fiber length compensate for an increase in afterload. When the afterload is first increased, the stroke volume ejected by the ventricles during systole is less than the filling volume. The consequent excess of volume in the ventricles stretches the myocardial fibers in the ventricular walls. This increase in myocardial fiber length enables the ventricles to eject a given stroke volume against an increased afterload. Intrinsic control of contractility Heart rate effects When the heart rate is suddenly increased, the force increases over the next several beats. This progressive increase in developed force induced by changing contraction frequency is known as the staircase phenomena. The initial rise in developed force when the interval between beats is suddenly decreased is achieved by a gradual increase in the intracellular calcium content. Intrinsic control of contractility Two mechanisms for the rising calcium: An increase in the number of depolarization per minute An increase in the inward calcium current per depolarization As the interval between beats is suddenly diminished, the inward calcium current progressively increases with each successive beat until a new steady-state level is attained at the new basic cycle length. Intrinsic control of contractility PVCs also affect the strength of contraction. When a PVC occurs, the premature contraction itself is feeble, whereas the beat after the subsequent pause is very strong. This response depends partly on the Frank Starling mechanism. Intrinsic control of contractility The beat is weak because not enough time has elapsed to allow much of the calcium taken up by the sarcoplasmic reticulum during the preceding relaxation to become available for release. Conversely, the postextrasystolic beat is considerably stronger than normal. The reason is that after the pause between beats, the sarcoplasmic reticulum had available for release the calcium had been taken up during two heartbeats: the extrasystole and the preceding normal beat. This effect is in addition to the increased preload from the PVC. Extrinsic control of contractility Sympathetic Changes contractility can be evoked by stimulation of the left stellate ganglia. Neurally released norepinephrine or circulating catecholamines interact with beta adrenergic receptors on the cardiac cell membrane. The peak pressure and the maximal rate of pressure rise [dP/dt] during systole are markedly increased. The duration of systole is reduced and the rate of ventricular relaxation is increased during the early phases of diastole. The briefer systole allows more time for diastole and hence for ventricular filling. Extrinsic control of contractility Parasympathetic Vagal stimulation decreases the peak left ventricular pressure, maximal rate of pressure development [dP/dt], and maximal rate of pressure decline during diastole. The acetylcholine interact with muscarinic receptors which inhibits adenyl cyclase. The consequent fall in cAMP diminishes the calcium conduction of the cardiac cell membrane, reduces phosphorylation of the calcium channels, and hence decreases myocardial contractility. The acetylcholine released from vagal endings can also inhibit norepinephrine release from neighboring sympathetic nerve endings. Extrinsic control of contractility Other hormones Cortisol Hydrocortisone potentates the cardiotonic effects of catecholamines. This may be mediated in part by inhibition of the extraneuronal uptake of catecholamines. Thyroid hormones The rate of calcium uptake and of ATP hydrolysis by the sarcoplasmic reticulum are increased in response to excess thyroid hormones. Thyroid hormones increase protein synthesis in the heart which can lead to cardiac hypertrophy. These hormones also affect the composition of myosin isoenzymes in cardiac muscle. They increase principally those isoenzymes with the greatest ATPase activity, and thereby enhance myocardial contractility Extrinsic control of contractility Insulin Insulin has a predominant, direct, positive inotropic effect on the heart. Glucagon Effect of glucagon on the heart closely resemble those of the catecholamines. Both glucagon and catecholamines activate adenyl cyclase to increase the myocardial tissue levels of cyclic AMP. Extrinsic control of contractility pH, PaO2, PaCO2 Moderate degrees of hypoxia increase heart rate, cardiac output, and myocardial contractility via the sympathetic nervous system Severe degrees of hypoxia depress myocardial contractility. Neither the PaCO2 nor blood pH is a primarily determinant of myocardial function, intracellular pH matters. The reduced intracellular pH decreases the amount of calcium release from the sarcoplasmic reticulum in response to excitation. The diminished pH also decreases the sensitivity of the myofilaments to calcium. Any Questions?