enzyme
advertisement

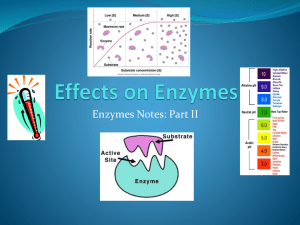
Chapter 6 (p. 97-105) and Chapter 20 (p. 375) 1. Demonstrate an understanding of the following terms: metabolism, enzyme, substrate, coenzyme, activation energy. (p. 102-105) 2. Identify the source gland for thyroxin and relate the function of thyroxin to metabolism. (p. 375) 3. Explain the “lock and key” model of enzymatic action. (p. 103) 4. Identify the role of vitamins in biochemical reactions. (p. 105) 5. Differentiate between the role of enzymes and coenzymes in biochemical reactions (p. 105) 6. Apply knowledge of proteins to explain the effects on enzyme activity of pH, temperature, substrate concentration, enzyme concentration, competitive inhibitors, and heavy metals. (p. 104-105) _____ Activation energy _____ Active site _____ Allosteric inhibitor _____ Apoenzyme _____ ATP energy _____ Catalyst _____ Co-enzyme _____ Competitive inhibitor _____ Concentration _____ Dehydration synthesis _____ Denature _____ Endothermic _____ Enzyme _____ Enzyme-Substrate Complex _____ Exothermic _____ Functional protein _____ Heavy metal ions _____ Homeostasis _____ Hydrogen carrier _____ Hydrolysis _____ Inhibitor _____ Iodine _____ Lock and key analogy _____ Metabolism _____ Optimum pH _____ Optimum temperature _____ Oxidize _____ pH _____ Structural protein _____ Substrate _____ Temperature _____ Tertiary structure _____ Thyroxin _____ Vitamin 1. There are approximately 3,000 of enzymes that exist in our body. Each of them with a specific function. 2. Enzymes help to promote metabolism, and help to catalyze every reaction that occurs in the body (ie: replication, transcription…). 3. Digestive enzymes also play a vital role in food digestion. 4. These enzymes act as sharp knives that cut up the complex foods into smaller units so that it can be assimilated easily into the blood stream. 5. The absorption will then enable our body to reassemble to build new cells and every other molecule our bodies need. • Since the tight control of enzyme activity is essential for homeostasis, any malfunction or mutation of a single critical enzyme can lead to a genetic disease. • A lethal illness can be caused by the malfunction of just one type of enzyme out of the thousands of types present in our bodies. Example #1: PKU. http://ca.youtube.com/watch?v=KUJVujhHxPQ Mutation in enzyme phenylalanine hydroxylase, which helps to digest phenylalanine, results in build-up of phenylalanine. This can lead to mental retardation . Can be controlled by a diet low in phenylalanine. Damage done is irreversible so early detection is crucial. Example #2: Mutations in genes coding for DNA repair enzymes. Will cause cancer since the body is less able to repair mutations in the genome. This causes a slow accumulation of mutations and results in the development of many types of cancer in the sufferer. An enzyme is a catalyst (a substance that speeds up a reaction without being consumed). Enzymes are proteins and are reusable. They work in low concentrations and speed up the reaction rate. Amylase Starch Lipase Lipids Glucose Fatty Acids and Glycerol Protease Proteins Amino Acids Enzymes allow reactions to proceed at lower temperatures than they would normally occur. The reactant(s) that an enzyme acts upon is known as the substrate(s). Enzymes work by forming a very temporary complex with the substrate. This is called the ENZYME SUBSTRATE COMPLEX. Enzymes are large globular proteins with very specific 3-D shapes (tertiary structure). Enzymes have grooves (or pockets), which may contain chemically functional groups. These areas are called active sites and this is where the substrate attaches. Specific groove shapes and chemical groups in an active site means that enzymes can only bond with one specific substrate or reactant. When the substrate and enzyme join together, the shape of the enzyme changes, which makes it more reactive. This is called INDUCED FIT http://www.phschool.com/science/biology_place /labbench/lab2/images/indfit.gif Many enzymes are made up of 2 pieces. 1) The APOENZYME – the protein portion (inactive) 2) The CO-ENZYME – a non-protein portion When these join together, the enzyme becomes active and the substrate will now ‘fit’ into the active site.. Co-enzymes usually fit into the ALLOSTERIC site, which changes the shape of the active site so the substrate can “fit.” The co-enzymes are often large molecules that the body cannot make on its own. Most co-enzymes are VITAMINS which we get from food or supplements. A typical balanced diet gives you all the vitamins you need, so there is little or no benefit from taking additional vitamin pills. The main exception to this is the vitamin folate, or folic acid, which is mainly found in dark green vegetables like spinach or collard greens. Not surprisingly, this is often deficient in the diet, and so in January 1999 the US government required companies making basic products like flour to add folate to the flour. So now when you eat bread, or pizza, or other common foods you are getting the folate your body needs. So pizza really is health food! The active site of an enzyme is not an exact perfect fit to the substrate. When the substrate attaches to the enzyme, this causes stress in the substrate, which will cause: 1.A substrate to break apart (in a hydrolysis reaction). Another word for this is CATABOLISM: breaking big molecules in to smaller ones. 2. Two substrates to form a bond (in a synthetic reaction). Another word for this is ANABOLISM: putting small molecules together to make bigger ones. •Definition: Metabolism is the constantly occurring chemical reactions that take place in a cell. •These chemical reactions occur in organized sequences from reactants to end products with the help of enzymes. •This organized sequence of reactions is known as a metabolic pathway. REACTANT Intermediate products PRODUCT Usually, heat is used to speed up chemical reactions by increasing the number of collisions that occur between reactants. Excessive heat, however, destroys the tertiary structure of protein (denatures it). Therefore, heat cannot be used to speed up reactions within living organisms. Enzymes operate by lowering the energy of activation needed for a reaction to occur. Enzymes act as a CATALYST and are not consumed in a reaction. This means that they can be used over and over again. 1. Concentration: The amount of enzyme and/or substrate available to react can affect enzyme activity. The reaction speeds up as the [substrates] increases, and it levels out when the enzymes are working at the maximum speed (saturation). What can you do to cause an increase in reaction rate? Add enzymes! The reaction speeds up as you increase the [enzyme], and slows down as the substrate has all been turned into product. What can you do to cause an increase in reaction rate? Add substrate! Enzyme Concentration 2. Temperature: As temperature rises, the reaction rate will increase as the enzymes and substrates bump into each other more often (kinetic molecular theory). At a certain point, the rate of these collisions will be at the fastest rate. This is the OPTIMUM TEMPERATURE. However, once you get above the optimum temperature, the enzyme becomes denatured (changes shape) and no longer functions properly. Most of our enzymes have an optimal temperature of 37oC (body temperature). The antarctic fish trematomus lives under the ice in the antactic ocean. It has large, bulging eyes to collect as much light as possible from the dim sea underneath the ice. The enzymes from these fish are so well adapted to cold environments that they denature (and the fish dies) if the temperature reaches only 5oC. As well as having enzymes that are adapted to the cold, these fish also have special glycoproteins that act as an antifreeze in their blood. This natural antifreeze is 300 times more effective than the antifreeze in your car. 3. pH: The 3D shape of an enzyme can be affected by pH. All enzymes have an optimal pH to work at depending on where they are in the body. • Saliva pH 7 • Stomach pH 2.5 • Intestines pH 8.5 • Vagina pH 2.5 When the pH is too low, the positive hydrogen ions interact with the negative R groups in the protein and tear them away. This denatures the enzyme by changing its shape. When the pH is too high, the negative hydroxide ions interact with the positive R groups in the protein and tear them away. This denatures the enzyme by changing its shape. H+ OH- When an animal dies, the body is decomposed by bacteria or fungi. If conditions prevent enzymes in the bacteria from working, the body will be preserved. This photo (Tollund man) shows a body that was discovered in Demark in a peat bog in 1950. The person had been strangled, and at first the police thought it was a recent murder. But peat bogs are very acid, and it turned out that the body was 2,000 years old, and had been very well preserved in the peat. Archaeologists believe the body is from a ritual murder, but they are not sure if the person was killed as a punishment, or whether the body was a sacrifice to the gods. 4. Inhibitors: Chemicals that interfere with the enzyme action. There are two types of INHIBITORS: a) Competitive Inhibitors b) Non-Competitive Inhibitors Allosteric Site Competitive Inhibitors are chemicals that so closely resemble an enzyme’s normal substrate that it can attach to the enzymes active site. The substrate and inhibitor “COMPETE”. If the inhibitor occupies the active site of the enzyme, the substrate will not be able to join and no product will form from that enzyme. If the Inhibitor is removed, the enzyme will become active again. Enzyme inhibitors are important commercially in many ways. For example pesticides kill bugs by inhibiting essential enzymes that are present in insects ( these enzymes are not found in humans) and herbicides kill weeds by inhibiting some of their important enzymes. Similarly many medications, such as aspirin and antibiotics are inhibitors. The enzyme substilin digests proteins, and is used in laundry detergent. Rennin, an enzyme extracted from calves, is used in curdling milk to make cheese. Glucose oxidase detects glucose in the urine (for example in diabetics). Non-Competitive Inhibitors are atoms or molecules that attach to an enzyme at an allosteric site and this denatures the enzyme. Non-competitive inhibitors will sometimes destroy an enzyme by permanently binding to the allosteric site. An example of this is heavy metals, such as lead in the nervous system. [substrate] Rate of Reaction Another type of non-competitive inhibition is when a metabolic product can feedback on a metabolic pathway to control how much product is made. The final product can temporarily attach to the allosteric site on the first enzyme. The enzyme will be denatured and the reaction will stop. http://ca.youtube.com/watc h?v=zl2KYhgZ_u8 This is an example of NEGATIVE FEEDBACK or FEEDBACK INHIBITION. When the concentration of the final product gets low again, there will be less inhibition on the enzymes and the metabolic pathway is reactivated. Thyroxin, the hormone that controls the metabolic rate of all of the cells in your body, is produced by the thyroid gland in the neck. If the concentration of thyroxin in your body is high, your metabolic rate will be raised, and if thyroxin levels are low, your metabolic rate will be low. The thyroid gland is stimulated to release thyroxin by a hormone produced in the pituitary gland called TSH (thyroid stimulating hormone). But the enzymes in cells of the pituitary that make TSH are inhibited by thyroxin. Therefore, if thyroxin levels are high, the pituitary stops producing TSH, and if thyroxin levels are low, the pituitary makes the TSH. Thus, the metabolic rate of cells in your body are maintained by the feedback inhibition of an enzyme http://ca.youtube.com/watch?v=VnneZReATW0 •Palpitations •Heat intolerance •Nervousness •Insomnia •Breathlessness •Increased bowel movements •Light or absent menstrual periods •Fatigue •Fast heart rate and trembling hands •Weight loss •Muscle weakness •Warm moist skin •Hair loss •Staring gaze Modern sloths live upside-down in the forests of South America and eat leaves in trees. They have claws to help them remain sleeping and suspended underneath branches for hours. A sloth's grip on its branch is so secure that in death it continues to hang unless it is forcibly unhooked. Sloths are generally nocturnal and move around little when awake. When they do move, it is at a slow and deliberate speed. Sloths have a very slow metabolism and take their sweet time digesting food (1-2 weeks per meal) and consequently, only defecate once in a one week period. Their metabolism is so slow that they may take a half a minute to move a leg a few inches. Their digestive system is so slow that they need only defecate about once a week. They even sneeze slowly. Being so slow, and indeed entirely immobile for much of the time, they are almost invisible to predators. By keeping such a low profile they avoid running into dangerous confrontations. http://ca.youtube.com/watch?v=eotEEUN atKY&feature=related However, sloths just don't get much done in life. Their birthrate is low, with a single young born once a year. They can't do much for their kids anyway -- a mother rushing to help her threatened infant was timed at 14 feet per minute. The slow or low rate of metabolism in sloths effects their ability to fight off illness. Most sloths have difficulty surviving when in captivity outside of their natural range because they cannot fight off new diseases or adapt to a colder climate. Most cell reactions (metabolism) require energy to occur. The energy ‘currency’ of cells is a molecule called ATP. ATP has 3 phosphates, the last two of which are held together by a high energy bond. It takes a lot of energy to make this phosphate bond, and energy is released when the bond is broken.