Outside of cell
advertisement
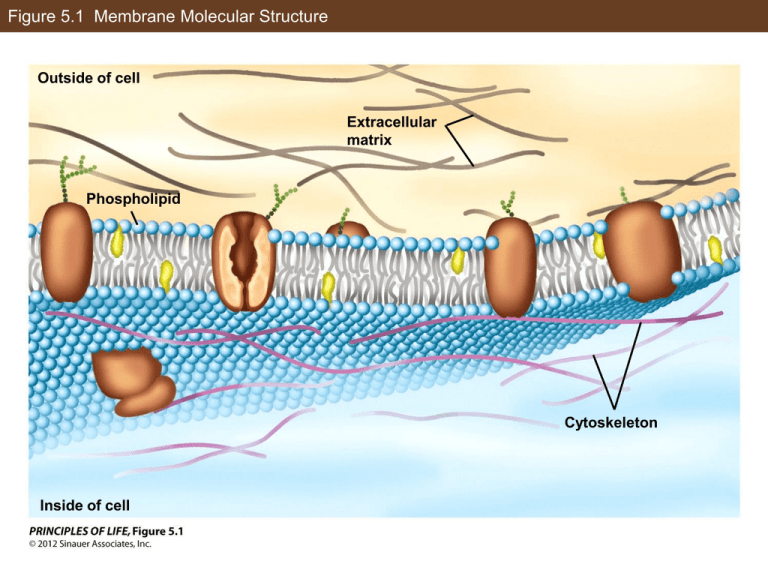
Figure 5.1 Membrane Molecular Structure Outside of cell Extracellular matrix Phospholipid Cytoskeleton Inside of cell In-Text Art, Ch. 5, p. 64 “Head” “Tails” In-Text Art, Ch. 5, p. 65 Outside of cell (aqueous) Hydrophobic interior of bilayer Inside of cell (aqueous) In-Text Art, Ch. 5, p. 66 Cells Figure 5.2 Rapid Diffusion of Membrane Proteins (Part 1) Proteins embedded in a membrane can diffuse freely within the membrane. Membrane proteins Mouse cell Human cell Figure 5.2 Rapid Diffusion of Membrane Proteins (Part 2) Proteins embedded in a membrane can diffuse freely within the membrane. Membrane proteins Mouse cell Human cell Heterokaryon Membrane proteins can diffuse rapidly in the plane of the membrane. Figure 5.2 Rapid Diffusion of Membrane Proteins (Part 3) The experiment was repeated at various temperatures with the following results: Temperature (C) 0 15 20 26 Cells with mixed proteins (%) 0 8 42 77 Plot these data on a graph of Percentage Mixed vs. Temperature. Explain these data, relating the results to the concepts of diffusion and membrane fluidity. Figure 5.3 Osmosis Can Modify the Shapes of Cells (Part 1) Hypertonic on the outside (concentrated solutes outside) Inside of cell Outside of cell Isotonic (equivalent solute concentration) Hypotonic on the outside (dilute solutes outside) Figure 5.3 Osmosis Can Modify the Shapes of Cells (Part 2) Hypertonic on the outside (concentrated solutes outside) Animal cell (red blood cells) Isotonic (equivalent solute concentration) Hypotonic on the outside (dilute solutes outside) Figure 5.3 Osmosis Can Modify the Shapes of Cells (Part 3) Hypertonic on the outside (concentrated solutes outside) Plant cell (leaf epithelial cells) Isotonic (equivalent solute concentration) Hypotonic on the outside (dilute solutes outside) Figure 5.4 A Ligand-Gated Channel Protein Opens in Response to a Stimulus Outside of cell Stimulus molecule (ligand) Binding site Hydrophobic interior of bilayer Channel protein Closed channel Inside of cell Hydrophilic pore Figure 5.5 Aquaporins Increase Membrane Permeability to Water (Part 1) Aquaporin increases membrane permeability to water. Aquaporin mRNA Aquaporin channel Protein synthesis Figure 5.5 Aquaporins Increase Membrane Permeability to Water (Part 2) Aquaporin increases membrane permeability to water. Aquaporin mRNA Aquaporin channel Protein synthesis 3.5 minutes in hypotonic solution Aquaporin increases the rate of water diffusion across the cell membrane. Figure 5.5 Aquaporins Increase Membrane Permeability to Water (Part 3) Oocytes were injected with aquaporin mRNA (red circles) or a solution without mRNA (blue circles). Water permeability was tested by incubating the oocytes in hypotonic solution and measuring cell volume. After time X in the upper curve, intact oocytes were not visible: Relative volume X With mRNA Without mRNA Time (min) A. Why did the cells increase in volume? B. What happened at time X? C. Calculate the relative rates (volume increase per minute) of swelling in the control and experimental curves. What does this show about the effectiveness of mRNA injection? Figure 5.6 A Carrier Protein Facilitates Diffusion (Part 1) Outside of cell High glucose concentration Glucose Glucose carrier protein Inside of cell Low glucose concentration Rate of diffusion into the cell Figure 5.6 A Carrier Protein Facilitates Diffusion (Part 2) Glucose concentration outside the cell Figure 5.7 Primary Active Transport: The Sodium–Potassium Pump Outside of cell High Na+ concentration, low K+ concentration Na+ Na+– K+ pump K+ K+ ATP Na+ Pi ADP Pi Pi Pi K+ Inside of cell High K+ concentration, low Na+ concentration Figure 5.8 Endocytosis and Exocytosis (Part 1) (A) Endocytosis Outside of cell Plasma membrane Inside of cell Endocytotic vesicle Figure 5.8 Endocytosis and Exocytosis (Part 2) (B) Exocytosis Secretory vesicle Figure 5.9 Receptor-Mediated Endocytosis (Part 1) Outside of cell Specific substance binding to receptor proteins Cytoplasm Clathrin molecules Coated pit Coated vesicle Figure 5.9 Receptor-Mediated Endocytosis (Part 2) Outside of cell Specific substance binding to receptor proteins Coated pit Cytoplasm Coated vesicle Clathrin molecules Figure 5.10 Chemical Signaling Concepts Secreting cell Receptor Target cell Target cell Circulatory vessel (e.g., a blood vessel) Target cell Figure 5.11 Signal Transduction Concepts Signal molecule Receptor Short-term responses: enzyme activation, cell movement Inactive signal transduction molecule Activated signal transduction molecule Long-term responses: altered DNA transcription Figure 5.12 A Signal Binds to Its Receptor Ligand Outside of cell Cell membrane Inside of cell In-Text Art, Ch. 5, p. 76 Signal molecule Receptor R+L RL Figure 5.13 A Protein Kinase Receptor Signal (insulin) Outside of cell Receptor Protein kinase domain (inactive) ATP ADP Phosphate groups Target Cellular responses Inside of cell Figure 5.14 A G Protein–Linked Receptor (Part 1) Outside of cell Signal (hormone) GDP G proteinlinked receptor Inside of cell Inactive G protein Inactive effector protein Figure 5.14 A G Protein–Linked Receptor (Part 2) Outside of cell GTP Activated G protein Inside of cell Figure 5.14 A G Protein–Linked Receptor (Part 3) Outside of cell Activated effector protein GDP Reactant Inside of cell Product Amplification Figure 5.15 The Discovery of a Second Messenger (Part 1) A second messenger mediates between receptor activation at the plasma membrane and enzyme activation in the cytoplasm. Cytoplasm contains inactive glycogen phosphorylase Liver Membranes contain epinephrine receptors Figure 5.15 The Discovery of a Second Messenger (Part 2) A second messenger mediates between receptor activation at the plasma membrane and enzyme activation in the cytoplasm. Active glycogen phosphorylase is present in the cytoplasm. A soluble second messenger, produced by hormone-activated membranes, is present in the solution and activates enzymes in the cytoplasm. The activity of previously inactive liver glycogen phosphorylase was measured with and without epinephrine incubation, with these results: Condition Enzyme activity (units) Homogenate Homogenate + epinephrine Cytoplasm fraction Cytoplasm + epinephrine Cytoplasm + membranes Cytoplasm + membranes + epinephrine 0.4 2.5 0.2 0.4 0.4 2.0 A. What do these data show? B. Propose an experiment to show that the factor that activates the enzyme is stable on heating and give predicted data. C. Propose an experiment to show that cAMP can replace the particulate fraction and hormone treatment and give predicted data. Figure 5.16 The Formation of Cyclic AMP (Part 1) Adenylyl cyclase ATP cAMP + PPi Figure 5.16 The Formation of Cyclic AMP (Part 2) Adenine Phosphate groups ATP Figure 5.16 The Formation of Cyclic AMP (Part 3) Cyclic AMP (cAMP) Figure 5.17 A Cascade of Reactions Leads to Altered Enzyme Activity (Part 1) 1 Epinephrine Outside of cell Activated G protein subunit Epinephrine receptor Plasma membrane Activated adenylyl cyclase GTP ATP cAMP 20 Inactive protein kinase A 20 Active protein kinase A Inactive phosphorylase kinase 100 Active glycogen synthase Inactive glycogen synthase Active phosphorylase kinase Figure 5.17 A Cascade of Reactions Leads to Altered Enzyme Activity (Part 2) 100 Active phosphorylase kinase Inactive glycogen phosphorylase 1,000 Active glycogen phosphorylase Glycogen 10,000 Glucose 1-phosphate Glucose Inside of cell 10,000 Outside of cell Blood glucose Figure 5.18 Signal Transduction Regulatory Mechanisms (Part 1) ATP Protein kinase P Inactive enzyme Active enzyme Pi Protein phosphatase Figure 5.18 Signal Transduction Regulatory Mechanisms (Part 2) Receptor binding Inactive G protein GTP GDP GTPase Active G protein Figure 5.18 Signal Transduction Regulatory Mechanisms (Part 3) Adenylyl cyclase ATP Phosphodiesterase cAMP AMP Figure 5.19 Caffeine and the Cell Membrane (Part 1) Outside of cell Plasma membrane Inside of cell Figure 5.19 Caffeine and the Cell Membrane (Part 2) Caffeine Adenosine