Tomography of flagellar
pocket
Lacomble et al., J Cell Sci. 122:1081-90.
Sleeping Sickness and
Trypanosomes
Trypanosomias
David Bruce, 1855-1931
Trypanosomes were first
described in frogs 1855
Griffith Evans identifies T.
evansi as agent of surra (a
horse and camel disease) in
1880
David Bruce identifies T.
brucei as cause of Nagana
and demonstrates
transmission by Tse-tse
flies
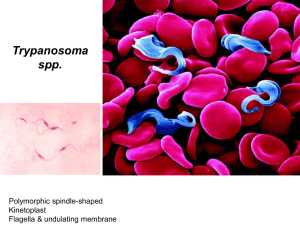
Trypanosome biology
The kinetoplast consists of a complex
network of concatenated DNA cirlces
Mensa-Wilmot lecture will tell you what the circles are for
Trypanosome biology
Tse tse flies
Parasites are taken up with
the blood meal (stumpy forms
are cell cycle arrested and
‘ready to go’ for the next host
Transformation into procyclic
trypomastigotes in the midgut
Migration into the
ectoperitrophic space where
parasites replicate
Passage into salivary glands,
differentiation into
epimastiogotes which attach to
the epithelium and massively
replicate
Transformation into infectious
metacyclic trypomastigotes
Again these are cell cycle
arrested ‘sleepers’
Trypanosmes have sex and likely
it happens in the salivary gland
Genetic exchange occurs (e.g.
double drug resistance occurs after
coinfection of fly with single
resistant parents)
Genetic exchange/sex is likely not
obligatory to complete fly
development and population
genetics suggest “modest” sex
Nobody has seen it, yet it likely
involves fusion and meiosis
(progeny appears largely diploid
but there are also polyploids)
Most likely this exchange/fusion
occurs among detached salivary
gland epimastigotes
Trypanosmes have sex and likely
it happens in the salivary gland
GFP & RFP only parents early
on in salivary gland
Red, green & yellow progeny
Red, green & yellow progeny
Gibson et al., Parasites & Vectors 2008, 1:4
Insect stages and blood
stream forms are very different
Different stages express
different sets of surface
proteins
Insect forms have large
mitochondria with many
cristae
Insect stages have an aerobic
metabolism and a full
respiratory chain
Blood stream forms only
engage in glycolysis and
excrete pyruvate and glycerol
Note that transmission stages
do not replicate and are
arrested in development
(ladies in waiting)
Several species of trypanosomes cause
disease in domestic animals and man
T. brucei rhodesiense & gambiense cause sleeping
sickness
T. brucei brucei, T. congolense and T. vivax cause
Nagana in cattle
T. equiperdum causes sexually transmitted disease in
horses and camels (interestingly, T. equiperdum is a
recent ‘petite’ mutant of T. brucei (loss of mitochondrial
genome or kDNA) Lai et al. 2008, http://www.pnas.org/content/105/6/1999.full
Loss of oxidative phosphorylation locks parasite into BS
form – or the other way around, leaving Africa and tsetse transmission makes the mitochondrion dispensable
(There are trypanosomes infecting many species of
animals and even plants and every single deer in the
State of Georgia)
Sleeping sickness in man
Sleeping sickness in man
Trypanosomes multiply
in the tissue around the
initial bite site
This often results in a
characteristic local
inflamation the
trypansomal chancre
From there they enter
the blood and lymphatic
system
Sleeping sickness in man
Enlargement of the
lymphatic glands
(especially in the
posterior triangle of the
neck) can be an early
sign of the diseasese
(Winterbottom sign, not
as common in
rhodesiense infection).
Aspiration of swollen
gland often reveals
parasites.
Sleeping sickness in man
Once parasites enter blood
stream fever sets in (low and
irregular in gambiense and
high and periodic in
rhodesiense
General toxic symptoms
include headache, facial
oedema, nausea and
vomiting,back and bone pain
Symptoms at this stage are
rather mild in gambiense but
can be servere in
rhodesiense with often fatal
outcome
Sleeping sickness in man
The second stadium of
trypansomiasis is
characterized by progressive
anemia and kachexia.
Both features are primarily
due to extremely high serum
levels of TNFa
TNFa was isolated both as
factor with tumor necrotic
effect as well as kachexin
inducing wasting in nagana
Sleeping sickness in man
In later stages of infection
parasites pass the blood
brain barrier and infect the
CNS
Presence of parasites
leads to meningoencephalitis with
progressive neurological
involvement, which
ultimately ends in coma
(sleeping sickness)
Untreated trypanosomiasis
is always fatal
Sleeping sickness in man
The progressive encephalitis
can cause severe dementia
with sometimes aggressive
behavior
Disease progression especially
CNS invasion is much faster in
rhodesiense
Gambiense can take a year or
two rhodesiense usually
passes the blood brain barrier
within a month
Nagana is the major impediment
to cattle production in Africa
Almost the entire area
of subsaharan Africa
which is suitable for
cattle is Tsetse
infested
High losses due to
anemia and cachexia
especially in
productive breeds
Wild animals are important reservoirs
for human and cattle trypanosomiasis
Why is trypanosomiasis so
deadly?
Trypanosomes are highly
susceptible to antibodies
and complement
They live fully exposed to
antibodies in the blood
stream
They induce a very
strong antibody response
Still they manage to
thrive in the same host
for a year or longer
Why is trypanosomiasis so deadly?
Infection is
characterized by
periodic waves of
parasitemia
Why is trypanosomiasis so deadly?
Infection is
characterized by
periodic waves of
parasitemia
Each wave represents
a single antigenically
distinct clone or
serotype
Antigenic variation
The entire trypanosome
population seems
antigenically uniform but
at a very low frequency
divergent (so called
switched) serotypes are
encountered
Antigenic variation
Trypanosomes are
covered with a dense
surface coat
Variant specific
antisera strongly
react with this surface
coat
Surface coats from
different clones are
antigenically distinct
The surface coat consists of
a single 65 kDa glycoprotein
A single protein can be
labeled on the surface
of trypanosomes
Upon parasite lysis this
protein becomes
soluble and can be
purified to homogeneity
fairly easily.
George Cross
http://tryps.rockefeller.edu/
Different antigenic variants have
different surface glycoproteins
VSGs from different clonal
isolates have the same
molecular weight but vastly
different amino acid
compositions
Vaccination with a given
VSG protects against
challenge with the
homologous isolate but not
against another variant.
VSGs share a common
structure
All VSGs are 65 kDA
glycoproteins
Most contain classical N-linked
glycans and all are anchored via a
GPI glycolipid (cross reacting
determinant)
Two domains can be cleaved by
trypsin
The outer domain is highly
variable and the only conservation
detected is the position of
cysteines
VSG forms dimers
Antigenic variation
VSG dimers form a densly packed surface coat
Other (non-variant) proteins like transferrin receptor or
hexose transporter are hidden in this coat
Trypansomes harbor ~1000
different VSG genes
The genomic organization of
trypanosomes is quite complex with
20 chromosomes and 100 mini
chromosomes
Great variability of chromosome
size between isolates
6-10% of the total DNA is coding for
VSGs (~1000 genes)
Only one is expressed
3 very peculiar details emerged
from studying the mRNA of VSG: all
trypanosome mRNAs seem to have
the same 5’end, and the VSG
mRNA encodes a hydrophobic cterminus absent from the mature
protein sequence, VSG message is
transcribed by Pol I
Antigenic variation
mRNA derived from only a single VSG gene
can be detected at one time
VSG expression is controlled at the level of
transcription initiation
Regulation of promoter activity is used to
control gene expression in many organisms
Transcription in trypanosomes
is polycistronic
But, only very few promoters
have been identified in
trypanosomes and they did not
seem to regulate the expression
of VSG
Also surprisingly transcription in
trypanosomes was found to be
polycistronic
Polycistronic means that a
number of genes are transcribed
at the same time into one long
messenger RNA
In bacteria this message is
translated into protein, in
trypanosomes further
processing is needed
Transcription is polycistronic
The 39 first (5’) base pairs of
all trypanosme mRNAs are
identical, this sequence is not
found in the genomic locus of
these genes
Individual mature mRNAs are
derived from large
polycistronic transcripts and
short SL-RNAs by transsplicing (details in MensaWilmot lecture)
This might help control – but
was shown not to be the key to
antigenic variation
Location in the genome?
VSGs are expressed from telomeric
polycistronic expression sites
Active VSG genes are typically at the “ends” of
chromosomes (telomeres)
They are found in “expression sites”
Genes are read in (~20) expression sites like tapes
in a tape recorder but only one recorder is playing
at a time
How do you get a new tape in and how are the
recorders controlled e.g. switched on and off?
Several mechanisms for
switching have been described
Antigenic variation
Transposition of VSG
genes occurs by intraor intermolecular
recombination
This explains switching
but not really why one
gene is active and all
the others are silent
Antigenic variation
Regulation could be achieved by
modification of chromatin
Indeed active and inactive sites differ in
the amount of a special modified base
called J (b-glucosyl-hydroxy-methyluracil a T variant) and there are newly discovered
differences in histone methylation and
acetylation patterns (Bob Sabatini will go over this
in detail on Monday)
For the next experiment we
need a mushroom
Amantia bisporingea, the
Destroying Angel
http://www.mushroomexpert.com
VSG is transcribed by Pol I
tubulin
rRNA
Drug
VSG
a-amanitin is a specific and highly
potent RNA polymerase inhibitor
Cells have specialized RNA
polymerases to transcribe different
genes
In most cells mRNA which encodes
proteins is transcribed by the RNA
polymerase Pol2 (this enzyme can be
inhibited by the toxin a amanitin)
Ribosomal RNA is generally
transcribed by Pol1 (which is resistant
to the toxin)
VSG transcription is insensitive to aamanitin suggesting it is transcribed by
the highly processive Pol I (however all
other mRNAs for proteins seem to be
made using Pol II as everywhere else)
How could this help to explain allelic
exclusion?
African trypansome cellular
architecture
Nucleus
Nucleoulus
Kinetoplast
How is a single expression
site activated?
Location, location,
location
PolI antibody detects
two spots in blood
stream forms: the
nucleolus (where
rRNA is made) and a
second locus outside
of the nucleolus
Navarro M, Gull K. Nature 414:759-63
How is a single expression
site activated?
The additional spot of PolI is not the nucleolus
Navarro M, Gull K. Nature 414:759-63
How is a single expression
site activated?
control
The extranuclear
PolI structure is
transcriptionally
active
a-amanitin
a-amanitin
Navarro M, Gull K. Nature 414:759-63
How is a single expression
site activated?
active VSG
inactive VSG
Labeling of the expression sites using GFP-Lac
Active, not inactive VSG expression sites colocalize
with the extranuclelarPolI spot
Navarro M, Gull K. Nature 414:759-63
Antigenic variation
Only a single VSG gene out of ~1000 is
expressed
Expression occurs out of teleomeric expression
sites (the tape recorder)
To switch genes on they are transposed into an
active expression site by several mechanisms
Expression seems promoter independent
Inactive DNA is modified
Expression seems to be controlled by physical
association of ES with a single POL1 transcription
particle per nucleus