Presentation - Cornell Institute for Biology Teachers
advertisement

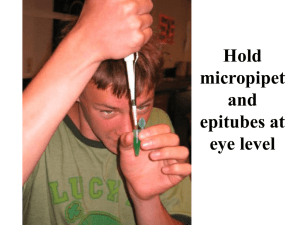
DNA Electrophoresis Cornell Institute for Biology Teachers What is this? How much do you know about it? http://www.britannica.com/eb/art-40216/DNA-molecule What are changes in DNA called? 1. Who thinks that DNA changes a lot? 2. Who thinks that DNA doesn’t change much? You are both correct. Although large portions of human DNA remain largely unchanged, other portions of the molecule can change A LOT. We know that ONLY about 5% of the genome codes for proteins that make up our body. The remaining 95% of the DNA has been called ‘junk DNA’ because it doesn’t code for any proteins. However, we are learning that it has regulatory functions: some of this regulates how genes behave and what genes do. Still, it is this 95% that changes a lot and is different in every person. This is the part that we are going to be most interested in, and will show us how unique each human is. http://www.rps.psu.edu What are chromosomes? Chromosomes are rod-shaped structures made of DNA and proteins that become visible in the cell nucleus when cells begin to divide. http://micro.magnet.fsu.edu What are genes? Hereditary units consisting of a sequence of DNA Occupy a specific location on a chromosome Determine a particular characteristic in an organism. Genes undergo mutation when their DNA sequence changes. http://publications.nigms.nih.gov In the nucleus of the cell in eukaryotes. Where is DNA found? In prokaryotes it is in the cytoplasm – no nucleus! http:// cmweb.pvschools.net http://content.answers.com Does every cell in the human body have a nucleus? Red blood No! cells have nucleus in Redablood cells thedon’t early have stagesaof development, but nucleus. expel them as they mature to make room for the So how do hemoglobin they carry (remember: they the main function of reproduce? red blood cells is oxygen and carbon dioxide transportation, in which hemoglobin is involved). www.irondisorders.org How big is the human DNA? If you take one cell, and stretch all 46 chromosomes end on end, the resulting string of DNA is about 2 meters long. What can we do to study such a long molecule? Restriction Enzymes More than 900 known Isolated from bacteria Recognize a particular sequence and cut in a specific position within that sequence Their names derive from the organism from which they were isolated Example: EcoRI (a.k.a ‘eco R one’) Isolated from certain strains of E. coli Recognizes the sequence G-A-A-T-T-C Cuts between the G and the A DNA fragments Gel Electrophoresis Steps Agarose gel fibers 1. Cut DNA 2. Make gel 3. Run DNA in the gel Gel Electrophoresis DNA fragments 1. Why does the DNA move? 2. How do we know how far the DNA has run? 3. When do you stop running the gel? 4. Why do differently sized fragments of DNA move at different speeds? Gel Electrophoresis DNA fragments Agarose gel fibers - - + + - + 1. The DNA is negatively charged by nature, and it moves because it is attracted to the positive end of the gel box. 2. To make sure the DNA doesn’t run off the gel we use a loading (or indicator) dye. The dye is a small molecule, much faster than any of the DNA fragments and is visible to the naked eye. Because it runs faster than the DNA fragments, we can be sure that once it reaches the end of the gel, the DNA fragments are still in the gel. 3. When the loading dye reaches the end of the gel, we know the DNA is close to the edge as well. 4. The smaller fragments of DNA move faster than the heavier, larger fragments because it is Gel Electrophoresis: Paternity Case #1 Mother 1 Child 1 2 3 Suspect 4 5 Are we sure this is not the biological father, or do we need more testing? Which is the most interesting subject in a paternity case? mother This child inherited band #1 from:______________ This child inherited band #2 from:______________ Biological father Gel Electrophoresis: Paternity Case #2 Mother Is the suspect the BIOLOGICAL father of the baby, or do we need further testing? 1 Child Suspect 1 3 2 2 4 This child inherited band #1 from:_____________ mother This child inherited band #2 from:______________ Biological father Divide the class in working groups of 4 Make sure everyone can see the front! Have you seen a pipettor before? Look at the top of your pipettor: > 2 20 This is the range that your pipettor can handle. What is the unit? Microliter (µl) 1 Decimal point 5 0 > microliters Practice! 1. Set your pipettor to: 0 7 How much is this? 7 µl 0 2. Now push the control button and find the first stop. 3. Keep pushing and find the second stop. Become familiar with these stops! How to insert a pipette tip 1. Open the pipette tip box 2. Insert the pipettor into the tip, and push 3. Take the pipette and tip out of the box and secure with your fingers by pushing it backwards. Do not touch the front end of the tip! Pipettor use and safety guidelines Pipettor Demo! Teacher transfers 200 µl of green colored water to another tube. - Always hold tube at eye level - Push control button to first stop and hold - Submerge tip in the solution and let go of control button (you are sucking up the solution) - Transfer to second tube: go to side wall of the second tube with the pipette tip and push control button to the first stop; hold for a second, then push all the way to the second stop - Holding down the control button, take out the pipettor. If you don’t hold the control button down before you take the pipettor out, you will suck up some of the solution again More Practice Pipettor Practice! Working with a partner: 1. Take a square of parafilm 2. With the pipettor set at 7 ul, make a line of 5 drops of green colored water on one side of the parafilm. Remember to go to the first stop and then submerge the tip in the solution! 3. Have your partner do the exact same thing on the opposite side of the parafilm 4. Compare the drops. If they are all same size you must have used the correct volume. What could have happened if not all the drops are the same size? 5. Wipe drops with with Kim wipes and discard 6. Push eject button and drop tip in the waste basket Teacher: collect water tubes! Divide each of the working groups of 4 into two teams of 2 students Make sure everyone can see the teacher! 1. Establishing Paternity Have each team of 2 take 6 clean Eppendorf’s from the baggie and set on their tube rack 2. We are going to label them on the top (lid), depending on which case each team gets. Case 3 3. Decide which team gets which case and label away! (*) Case 4 Teacher hands out M, B and S tubes, distilled water and buffer, but not the enzyme or standard yet! (*) will be supplied later. No tubes need to be labeled as ‘standard’ (*) Establishing Go to page 8 of your handouts. Paternity To analyze your DNA samples, they need to be cut using a restriction enzyme, in this case, HindIII. Are all samples going to get HindIII? What does the “+” stand for? All tubes must have the exact same volume: 20 µl Establishing Page 8 in your handouts. Paternity All tubes must have the exact same volume: 20 µl There are 6 columns to fill out. This is the list of ingredients. What are we going to add first? ____How much in each tube?___Remember, each tube should contain a maximum of 20 µl of biologicals! Fill out the chart. Establishing Page 8 in your handouts. Paternity All tubes must have the exact same volume: 20 µl Double check your amounts! How do “13 µl” look like in the pipettor’s 1 window? 3 0 How do “12 µl” look like in the pipettor’s window? 1 How do “2 µl” look like in the pipettor’s window? 0 2 2 How do “5 µl” look like in the pipettor’s 0 window? 5 0 0 0 How does “1 µl” look like in the pipettor’s window? 0 1 0 Establishing Page 8 in your handouts. Paternity All tubes must have the exact same volume: 20 µl When pipetting, make sure you SEE what you are adding to the tubes! Put the substance on the side wall of the Eppendorf (we will centrifuge it later) Choose one person to add the enzyme to the “+” tubes ONLY!!! If no enzyme is added the lab WILL NOT WORK!! DO NOT FORGET TO CHANGE THE PIPETTE TIPS TO AVOID Establishing Page 8 in your handouts. Paternity All tubes must have the exact same volume: 20 µl GO AHEAD AND FILL UP ALL THE TUBES, one by one, following the teacher’s instructions!!!! Establishing Page 9 in your handouts. Paternity Next, one team member brings the tubes in the tube rack to the centrifuge, closes the lid and centrifuges for 2 seconds and then….. …..puts the tubes in the incubator. Pick a letter (on the side of the incubator) to identify the team’s tubes. Stick with this letter so your tubes can be retrieved later! After all tubes are in the incubator, ask a volunteer to put the rest of the Eppendorf tubes (DNA samples, HindIII, etc.) in the waste basket! Establishing Paternity Page 9 in your handouts. How long do samples need to be incubated? Don’t forget to keep track of the time!!!! 37 ˚ C At what temperature? Why this temperature? Where do restriction enzymes come from? What reaction is taking place in the Eppendorf tubes right now? DNA is being cut in smaller fragments Would the reaction take place at room temperature, at about 20 ˚ C? Probably yes, but more slowly Would the reaction take place if the temperature was about 75 ˚ C? Probably not; the enzyme would be denatured Establishing Paternity Think about the enzymatic reaction taking place in the incubator…. What would a graph illustrating this reaction look like: A, B or C? Percentage of cut DNA (A) (B) 100% 50% (C ) Time Establishing Paternity Think about the enzymatic reaction taking place in the incubator…. What would a graph illustrating this reaction look like: B Percentage of cut DNA What would be the time at which the reaction is completed? 100% (B) 50% 30 min Time Establishing Page 9 in your handouts. Paternity What is the next step in the lab? Prepare the buffer!Tables with the big flask also have a plastic bottle with concentrated buffer. 1.Add 1900ml of distilled water to the flask 2. Take 100 ml from the buffer bottle and add to the big flask with the 1900 ml of distilled water. 3. Groups with the smaller 1000 ml flask bring your flasks to groups with the 2000 ml flasks. Mix back and forth 3 times and then take 1000 ml of the solution. 2000 ml is enough for 2 gels! NO SPILLS! 4. The rest of the concentrated buffer in the bottle (100 ml) can be used for two more gel boxes in the same fashion. Establishing Page 9 in your handouts. Paternity What is the next step in the lab? Prepare the agarose! Measure 1 gram of agarose in a paper cup. Take into account the weight of the cup (tare or rezero)! Transfer the agarose to the small 250 ml flask. Measure 100 ml of buffer solution in the graduated cylinder and add to the flask with the agarose. Bring the flasks to the microwave oven and follow teacher’s instructions. Memorize the label on your flask so you can recognize it later!! Let it boil for a minute or so until the agar is totally dissolved. Establishing Page 9 in your handouts. Paternity What is the next step in the lab? Preparing the agarose…. After boiling, set all the flasks in a line…. …and let them cool down until the temperature reaches 75 ˚ C…. The teacher will add 2 µl of GelStar to each flask. Swirl the solution. Establishing Page 9 in your handouts. Paternity What is the next step in the lab? Preparing the gel boxes 1. Slide the top sideways and find and remove the tray and the comb tray comb 2. Firmly secure the gasket and create a leak proof chamber into which the liquid gel will be poured. Push down evenly so gaskets don’t pop out of the Establishing Page 9 in your handouts. Paternity What is the next step in the lab? Preparing the gel boxes Pour the gel inside the gel box, inside the leak proof chamber you just created. Look at the comb. Find the side with the thicker teeth and put this side of the comb in the gel. Use the grooves towards the end of the gel tray, not the ones in the center! Wash the Erlenmeyer flask right away, before the gel starts to solidify inside! Establishing Page 10 in your handouts. Paternity What is the next step in the lab? Loading the gels Bring the Eppendorf tubes back from the incubator. Look at the procedure, page 10. What are we still missing from the tubes? What do we still need to add to the tubes, so that we know when to stop the gel run? Loading dye How much do you add to each tube? 5 µl How many pipette tips do you need? 6, one for each Establishing Page 11 in your handouts. Paternity What is the next step in the lab? Loading the gels Add the dye, 5 µl per tube, then put the pipette tips and used dye tube in the waste basket. Bring all tubes to the centrifuge and spin for 5 seconds. Bring all tubes back to your team. While the tubes are in the centrifuge have a volunteer empty the waste baskets into the trash. Establishing Page 10-11 in your handouts. Paternity What is the next step in the lab? Loading the gels Look at the list of samples. Which of these are we missing? Establishing Page 11 in your handouts. Paternity What is the next step in the lab? Loading the gels How many wells are you going to need? 14 How many wells do you have in the gel? 20 So, be careful with the loading, or you might run out of wells. And if you make a mistake, make sure to record it in your lab handout!! Establishing Page 11 in your handouts. Paternity What is the next step in the lab? Loading the gels Visually divide the grayish gel into thirds. You will insert the pipette tip about one third into the opaque gel…. buffer level 1/3 1/3 1/3 Correct Incorrect Incorrect Establishing Page 11 in your handouts. Paternity Loading the gels After placing the tip about one third into the gel (or half way into the well), push the contents of the pipette to the first stop only and then HOLD the yellow button in place! Don’t let go until you are actually out of the buffer that covers the entire gel, or else you will suck the sample back into the pipette tip! 1/3 1/3 1/3 Correct Incorrect Incorrect (punctured) ??? ? Incorrect! Also punctured Establishing Page 11 in your handouts. Paternity Loading the gels To steady your arm, place on top of the counter…. 1/3 1/3 1/3 Also, have a classmate look at the gel from the side, to help guide you, and tell you when to STOP and let go of the sample. REMEMBER: Go in, push to first stop only, hold yellow button & pull out! Establishing Page 11 in your handouts. Paternity Loading the gels Also!!! Locate the red tape on the gel box. Your wells should be sitting on top of the red tape, in order to make them easier to see from above. After filling up the wells, this is what they would look like from above. Establishing Page 12 in your handouts. Paternity Loading the gels This is the default order in which the samples should be loaded on to the gel. Go to page 12 in your handout. Use it to take notes of what you are actually doing. You might have to deviate from this order if you make a mistake. That’s OK! Just make sure that you note what happened next to the corresponding well (on page 12). If you mess up, remember: you have an extra 6 wells in the gel. And you have 25 µl of each sample. Since you need to load only 10 µl of each Establishing Paternity Loading the gels - Let’s get Team #3 pipettes first! organized! One member will be the guide, looking from the side, guiding the student loading the sample. Switch roles after loading 3 or 4 samples, so the pipetting student now guides and the guide now loads. Meanwhile, Team #4 becomes the ‘helper’ team. One member writes down the order in which the samples are being loaded on Page 12. The other member pre-loads the pipettor: make sure that they have the right volume setting, get a new pipette tip for each sample and hand the loaded pipettor (with the correct sample in it) to the student loading in the right order! Set all pipettors to the correct volume: 10 µl (looks like this in the window): Decimal point 1 0 0 Establishing Paternity Loading the gels - Are the gels ready? How would you describe the gel? When the gel is OPAQUE, it means its ready! Getting the gel box ready Getting the tray out of the gel box: One student holds the gel box down, another student takes out the tray without touching the gel. If there is a team member with nothing to do: organize the samples in the right order in the tube rack, according to the order on page 11. Establishing Page 10-12 in your handouts. Paternity Getting the gel boxes Find the red tape on the gel ready box. Place the gel tray so that the comb gets to sit over the red tape. The wells are easier to see when placed over the red tape. Get the buffer (you should have about 900 ml in the flask). Fill the side chambers of the gel box with the buffer. There is a black fill line on one side. Fill to the black line. ** Make sure the red tape is closer to the black wire!! ** Establishing Page 10-12 in your handouts. Paternity Loading the gels Very important: Once you start loading the gels you can’t move the gel box. Find a comfortable place where you can load the gel; find a good angle for the loading student and for the guide. Carefully pull the comb out of the gel. If the gel sticks to the comb, use the blunt end of a pipette tip to push it down gently. Set the comb aside. Take turns so everybody loads some samples!! Begin loading the samples, 10 µl in each well, in the correct order. Make sure to note any changes to the loading order on your lab handout (page 12). Establishing Page 10-12 in your handouts. Paternity Loading the When you are done loading: gels -every student needs to copy the notes from page 12 -put all tubes in the waste basket, then empty the waste basket in the trash bin. -rinse out all the glassware Carefully put the lid back on the gel box. DON’T RATTLE IT. Untie the cords and push the plugs all the way into the power supply box . Make sure black goes to black and red goes to red. Turn on the power supply, and set the voltage to between 185 V and 195 V. Samples will run for about 80-90 minutes, until the loading dye is close to the opposite end of the gel. Establishing Paternity While the gels run… Why is the box foggy? Water electrolysis is taking place, breaking the water molecules into separate molecules of hydrogen and oxygen which are released from the buffer and fog up the box and the lid. Establishing Allele Paternity Allele frequency is the relative abundance of Frequencies an allele at a genetic place (locus) in a population. It is expressed as a proportion or a percentage. Victim’s blood Victim’s blood Evidence sample Evidence sample Suspect’s blood Suspect’s blood Frequency in population Chance of having these two alleles is: 0.10 2 x 0.1 x 0.02 = 0.004 0.02 4 out of 1000 people in this population will have this pattern. Frequency in population 0.05 In NYC, for example, with a population of 10,000,000 this means 40,000 will have this pattern Chance of having these two alleles is: 2 x 0.05 x 0.01 = 0.001 0.01 1 out of 1000 people in this population will have this pattern. Establishing Allele Paternity Frequencies Remember: allele frequency is the relative abundance of an allele at a genetic place (locus) in a population. It is expressed as a proportion or a percentage. Victim’s blood Evidence sample Suspect’s blood Frequency in population 0.06 Chance of having these two alleles is: 0.2 2 x 0.06 x 0.2 = 0.024 24 out of 1000 people in this population will have this pattern. The chance of having all six alleles from the three loci: 0.004 x 0.001 x 0.024 = 0.000000096 = 9.6 x 10-8 ≈ 1 x 10-7 Or about 1 in ten million people in this population will have this pattern. In the USA, for example, with a population of 300,000,000 it means that 30 persons will have this pattern. Establishing Paternity (for teacher reference) Have a volunteer write on the board the order in which the samples were loaded: M3- B3- S3- M3+ B3+ S3+ #2. Have a volunteer draw two bands for the mother. Should be much further down because they were cut (in red, just for clarification purposes) #3. Have a volunteer draw two bands for the baby: one should match with the mother (in green, just to clarify) STANDARD M4- B4- S4- M4+ B4+ S4+ STANDARD #1. What does the + mean? Enzyme added What does the - mean? Doesn’t have enzyme What difference in size is there between these two types of samples? Which are smaller? (The “+”. The “ – “ are bigger). Which would you find towards the top of the gel? The “ – “, because they were not cut. Have a volunteer draw where you would expect the bands for the first 3 samples (in yellow, just to clarify), toward the top of the gel, somewhere similar. #4. Have a volunteer draw lines for the suspect, assuming the suspect is not the father (in purple, just to clarify) #5. How many bands are we going to have for the standard (see page 6, there are 7 bands). This is the whole genome of the bacteriophage Lambda, cut with HindIII, and the sizes of these fragments are well known. #6. Ask another volunteer to draw lines for M4-, B4and S4-. This is uncut DNA, should be along the top of the gel, somewhere. #7. Repeat #2 and #3, and #4 assuming one of the suspect’s bands matches one of the baby’s. Establishing Paternity Ask: which of the bands are the most important? The baby’s because we are trying to find something out about the baby: B3+ and B4+. Ask: why do we run “ – “ if they are not important? They are the controls for the action of HindIII. What would it mean if all the bands, for all M, B and S are towards the top of the gel after electrophoresis? That they were not cut by the enzyme. M3- B3- S3- M3+ B3+ S3+ STANDARD M4- B4- S4- M4+ B4+ S4+ STANDARD x Does this mean the suspect is the father? No, just that he cannot be ruled out, you need more testing. Establishing Go to Page 13 in your lab handout Paternity The DNA standard provides DNA fragments of known sizes that can be used as a reference when trying to find the size of the unknown DNA fragments run on the gel. We know the size of the standard’s bands, based on the information we get from the manufacturer of the standard. The size of each standard band is directly related to the distance traveled from the well. We can now measure the distance for each of the seven standard bands. Establishing Go to Page 13 in your lab handout Paternity What you can do now is plot the size and distance traveled for each standard band. x Band size ( (23 kb) Standard curve kb) x (9 kb) x (6.6 kb) x (4.3 kb) x (2.3 x kb) (2 kb) x (0.6 kb) Distance traveled (mm) Establishing Paternity Go to Page 13 in your lab handout When trying to find the approximate size of an unknown DNA fragment, measure the distance that the fragment traveled in the gel (from the well to where the band ended up in the gel) and use the standard curve to determine the size of the fragment. x (23 kb) Band size ( kb) x (9 kb) Size ( kb) x (6.6 kb) x (4.3 kb) x (2.3 x kb) (2 kb) x x (0.6 kb) Distance traveled (mm) Establishing Paternity Getting the gels out Any allergies to latex? Turn everything off. Using gloves, one person will slide the gel off the tray and put on the blue surface of the dark reader, where it will be analyzed. Put the orange lid on top of the gel and turn the dark reader on. Make sure the room is darkened. Establishing You Paternity should have 14 lanes to Analyzing the gels, Case 3. look at. The brighter lanes (#7 and #14) represent your…….. Standard (1) What do M3-, B3- and S3have in common with M4-, B4- and S4-? They were uncut, they are further up on the gel. Find the M3+ and the B3+. Do they share a band? Find the B3+ and the S3+. Do they share a band? What does this mean? One band matches. The suspect can’t be ruled out. Further testing is required to find M3B3- S3M4- S3M3+ S3+ B4B3+ 1 1 Establishing Paternity We are now looking at Case 4. Analyzing the gels, Case 4. M3- Find the M4+ and the B4+. Do they share a band? Yes Look at the baby’s second band. This second band did not come from the mother. It came from the biological father. Does the suspect have this band? No What does it mean? There is no match in the suspect’s bands with the second band of the baby (which must have come from the biological father). This suspect is off the hook! B3- S3M3+ S3+ M4+ B4+ S4+ B3+ 1 1