Moving to the nucleus .....
Biased transmission of alleles or entire
chromosomes
Segregation distortion (meiotic drive,
selfish DNA)
Gametophytic effects in plants
Biased or unusual patterns of gene
expression
Maternal effect genes
Imprinted genes (parent-of-origin
expression bias)
Paramutation (allelic cross-talk &
silencing)
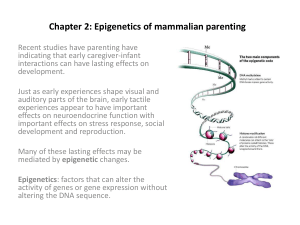
Epigenetics –
Heritable changes in gene expression that
do not involve changes in gene sequence
• Mediated by changes in chromatin structure
(Grelon & Elgin, Nature 447:399)
Epigenetics – Mediated by changes in
chromatin environment
e.g. Position effect variegation (PEV) in
Drosophila genes or transgenes located near
heterochromatic regions undergo mitotically
stable changes in expression due to changes in
chromatin organization
• An inversion placed the white
gene close to the pericentric
heterochromatin
• Sectors of drosophila white
gene off (white eye color)
result from heterochromatic
regions expanding to include
the white gene
How might this phenotype be used to investigate
the mechanisms of PEV and related gene
expression phenomena?
(Grelon & Elgin, Nature 447:399)
Epigenetics - Factors implicated in
heterochromatin formation
Repetitive DNA
DNA methyln
H3K9 methyln
HP1
Small RNAs
RDR
Sp
Nc
Dm
Mm
At
Yes
No
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
No*
No*
Yes
No*
Yes
Yes
Yes
No
Yes
Yes
Yes
Yes
Yes
No
Yes
Yes
Yes
No*
Yes
Yes
Yes - factor implicated in heterochromatin formation
No - factor is not present in the organism
No* - organism has the factor but no role in
heterochromatin formation
Sp, Schizosaccharomyces pombe; Nc, Neurospora
crassa; Dm, Drosophila melanogaster; Mm, Mus
musculus ; At, Arabidopsis thaliana
RDR, RNA-dependent RNA polymerase; HP1,
Heterochromatin Protein 1; Pol II, RNA polymerase II;
H3K9, Histone 3- lysine 9
(Grelon & Elgin, Nature 447:399)
Epigenetics - RNA silencing
mechanisms (RNAi)
• Transcriptional (chromatin changes)
• Post transcriptional
Transcript degradation
Translation repression
• Share common/core mechanistic features
A Nobel-prize winning discovery made in C.
elegans!
a) Silencing of a gfp transgene via doublestranded gfp RNA
b) Mutant defective in RNAi
[Fire et al. Nature 391: 806]
[Mello & Conte, Nature 431:338]
Epigenetics – RNAi mechanisms
• Post transcriptional
Transcript degradation
Translation repression
• Share common/core mechanistic features
• Note there are lots of routes to dsRNA
Nucleus
cytosol
[Buchon & Vaury Heredity 96:195]
Epigenetics – RNAi
• Transcriptional (chromatin changes)
Nucleus
[Buchon & Vaury Heredity 96:195]
Epigenetics - RNAi
• Transcriptional (chromatin changes)
• Post transcriptional
Transcript degradation
Translation repression
• Share common/core mechanistic features
• Present in a wide array of organisms
How are RNAi pathways adaptive or
advantageous? (To the organisms not the
molecular biologists!)
Epigenetics – Genomic imprinting
Modification of specific genes during
gametogenesis so that only the paternal or
maternal allele is expressed after fertilization,
i.e. “Parent of origin” gene expression
•
Seen in placental mammals and in plants
• For some imprinted genes, the maternal
allele is silenced & paternal allele
expressed
• For others, the paternal allele is silenced &
maternal allele expressed
Epigenetics – Genomic imprinting
Modification of specific genes during
gametogenesis so that only the paternal or
maternal allele is expressed after fertilization,
i.e. “Parent of origin” gene expression
• Affects the expression but not transmission
of alleles
• Two gene copies present
• One gene copy active = functional haploidy
Epigenetics – Genomic imprinting
Modification of specific genes during
gametogenesis so that only the paternal or
maternal allele is expressed after fertilization,
i.e. “Parent of origin” gene expression
A limited number of genes behave in this way
• ~80 of 30,000 human genes
• Primarily expressed in embryo and
placenta
• Plant imprinted genes are expressed in
the endosperm
• Almost no plant embryo expressed
imprinted genes
Epigenetics – Genomic imprinting
Early evidence from isoparental embryos in
mice
• created by manipulation of nuclei post
fertilization
• always lethal
• gynogenotes (2 egg-derived genomes)
have under-developed placenta
•
androgenotes (2 sperm-derived genomes)
have over-developed placenta
What does this say about the contributions of
each parental genome with respect to
development?
Genomic imprinting - Human Igf2-H19
a
Insulin growth factor 2 (Igf2) paternally
expressed
H19 (a non-coding RNA) maternally
expressed
Regulated by a differentially methylated
region (DMR, or imprinting control region ICR)
[MacDonald Genetics Res Intl doi:10.1155/2012/585024]
Genomic imprinting - Human Igf2-H19
Paternal DMRs methylated - H19 off, Igf2 on
• DMR1 is a silencer inactivated by
methylation
• DMR2 is an enhancer that is activated by
methylation
Maternal DMRs hypo-methylated - H19 on,
Igf2 off
• CTCF binds DMR
• Downstream enhancer engaged for H19
expression
• DMR1 silencer of Igf2 active
• DMR2 enhancer of Igf2 is inactive
[MacDonald Genetics Res Intl doi:10.1155/2012/585024]
Genomic imprinting - Human Igf2-H19
Practice question
In mice, complete loss of Igf2 function is viable, the
mutant mice are just much smaller than wild-type mice.
If a mouse is heterozygous for a loss-of-function
mutation at the Igf2 locus (genotype Igf2 – / + ), will this
mouse have a mutant or wild-type phenotype? Explain
your answer.
If an Igf2 – / + male mouse is mated with a wild-type (Igf2
+/+) female mouse, what are the expected frequencies
of Igf2 genotypes and resulting phenotypes in the
offspring? Explain your answer..
Genomic imprinting in mammals
Parent-of-origin gene expression imprints
must be correctly set in the gametes every
generation!
Female
embryo
Male
embryo
sperm
imprints
egg
imprints
meiosis
meiosis
• Maternal & paternal imprints are erased in
primordial germ cells (PGCs)
• Left, reset for a paternal pattern in sperm
• Right, reset for maternal pattern in eggs
• Failure to re-set correctly leads to
developmental disorders in next generation
[Kinoshita et al. Sem Cell Devel Biol 19:574]
Failure to correctly re-set imprints leads to
developmental disorders in next generation
e.g. Prader – Willi Syndrome (PWS) / Angleman’s
Syndrome (AS) locus
• ~ 6 mb deletion human 15q11– q13
• PWS patients (retardation, obesity) inherit 15q11–
q13 deletion from paternal parent (functional
maternal copy)
• AS patients (retardation, seizures) inherit deletion
from maternal parent (functional paternal copy)
• Some patients have two normal chromosomes but
both exhibit maternal expression pattern or paternal
expression pattern (imprinting re-set defect)
[ Horsthemke and Wagstaff, Am J Human Genet 146A:2041]
Genomic imprinting - Arabidopsis
endosperm (but not embryo)
PHE1 transcription factor
• master regulator of seed development
• paternally expressed
FWA, FIS2, MEA repressive chromatin re-modeling
proteins
• maternally expressed
Differential methylation established in gametophytes
• Paternal PHE1 on via 3’ methylation
• Paternal FWA, FIS2, MEA off via 5’ methylation
• Maternal FWA, FIS2, MEA on - demethylated by
DME
• Maternal PHE1 off - repressed by MEA-FIS2
chromatin remodeling complex
[Kinoshita et al. Sem Cell Devel Biol 19:574]
Epigenetics – Genomic imprinting
Parent-of-origin in gene expression
Plants and animals:
•
•
Genes involved in growth and development
Differential methylation (paternal vs maternal)
Plants:
•
•
•
Occurs in endosperm not embryo
Endosperm only (genetic dead-end) does not
require “re-setting” each generation
Genes are not clustered
Animals:
•
•
Occurs in placenta and embryo
Must be set for correct sex every generation
Complex loci
clustering of genes around Cis-acting
imprinting control (IC) regions
Clusters include paternally and maternally
expressed genes
Often anti-sense transcription of silenced
genes
Epigenetics – Paramutation
A change in expression of one allele
brought about by association with another
allele
How does this violate Mendel’s law of
independent segregation?
A paramutagenic allele is able to direct
change
A paramutable allele is susceptible to
change (paramutation)
After paramutation, the altered allele also
becomes paramutagenic
Paramutation is stable through at least
one (and sometimes many) subsequent
generations
A neutral allele is neither paramutable nor
paramutagenic
Most alleles are neutral!
Epigenetics – Paramutation at the B
locus of maize
B’- paramutagenic allele
conditions pale
pigment
B-I paramutable allele
conditions intense purple
pigment
F1 progeny are all pale!
How does this progeny differ
from Mendelian expectation?
BC1 progeny are all pale!
(Chandler and Stam, Nature Rev Genet 5:536)
Epigenetics – Paramutation at the B
locus in maize
Forward genetics provides answers!
Screen for mutants that maintain purple pigment in the
presence of B’
modifier of paramutation mop1-1 mutation
(A) B' Mop1+/mop1-1
(B) B' mop1-1/mop1-1
should look like A; instead looks like C
(C) B-I Mop1+/Mop1+
Positional cloning of the affected gene - mop1
encodes an RNA dependent RNA polymerase
What mechanism of paramutation is suggested by this
finding?
(Dorweiler et al. Plant Cell 12:2101; Alleman et al. Nature 42:295)
Epigenetics – Paramutation at the B
locus of maize
• B-I and B’ are identical in sequence
• B-I and B’ have 7 tandem repeated copies of an 853
bp sequence motif that is single-copy in neutral
alleles
• This motif is 100kbp upstream from the B promoter
• B-I has open chromatin conformation and B’ has
closed chromatin conformation in the repeat region
• Small RNAs transcribed from this repeat mediate
change in B-I chromatin conformation from open to
closed
• Important note: In paramutation, the direction of
the cross makes no difference!
[Arteaga-Vazquez & Chandler Curr Opin Genet Dev 20:156]
Epigenetics – Paramutation at the
mouse kit (tyrosine kinase) locus
Kit site-specific knock-out mutation by lacZ
insertion
(Kit tm1Alf/tm1Alf ) is homozygous-lethal
Heterozygote Kit tm1Alf/+ has white tail and
feet
[Rassoulzadegan et al. Nature 441:469]
Epigenetics – Paramutation at the
mouse kit (tyrosine kinase) locus
Kit +/+ x Kit tm1Alf/+
• All progeny white-tailed!
• Southern blot confirms recovery of
homozygous (Kit +/+) and heterozygous (Kit
tm1Alf/+) progeny
• Paramutation has altered expression of the
Kit+ allele, now designated Kit* +/+
Kit +/+ x Kit tm1Alf/+
[Rassoulzadegan et al. Nature 441:469]
Epigenetics – Paramutation at the
mouse kit (tyrosine kinase) locus
Kit tm1Alf/+ and Kit *+/+ :
• Reduced polyA Kit transcript relative to
Kit+/+
• Increased aberrant transcripts relative to
Kit+/+, including small RNAs
[Rassoulzadegan et al. Nature 441:469]
Non-Mendelian Genetics
Unusual patterns of gene transmission
• Organelle Genetics
• Segregation distortion
meiotic drive or “selfish genes”
Unusual patterns of gene expression
• Maternal effect genes
• Epigenetics
Small RNAs & gene silencing
Imprinting
Paramutation