Retinoblastoma
advertisement

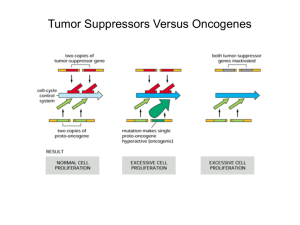
Tumor Suppressor Genes The story of Retinoblastoma Retinoblastoma is a cancerous disease 1/20,000 children; 300 per year Average age is 18 months Leukocoria or “white pupil” Treatment: enucleation = eye removal Prognosis is good after enucleation over 90% survival with early detection and treatment Rb is either sporadic or familial - Sporadic cancer in 55-65% of all cases - Sporadic cancers are unilateral Hereditary childhood cancer: - bilateral tumors in ~75% of cases - unilateral tumors in ~25% of cases Children with bilateral (familial) Rb have a high risk of developing non-retinal tumors Familial Sporadic Germ-line mutations in the Rb gene lead to predisposition to cancer In cancer patients with a family history of Retinoblastoma: the inheritance seems to be ? Rb tumors are associated with a deleted region in chromosome 13 Deletion = loss-of-function probably a recessive mutation in the Rb gene The Knudson’s “Two Hit” Hypothesis for the Generation of RB Alfred Knudson, PNAS 68:820 (1971) The Knudson’s “two hit” hypothesis for the generation of RB Retinoblastoma is inherited as a dominant trait, but it is recessive at the cellular level People with familial Retinoblastoma carry one mutated copy in ALL their cells. Cells that would get a second hit will develop Rb or later, other cancers Loss of heterozygosity (LOH) Loss of heterozygosity (LOH) in a cell represents the loss of normal function of one allele of a gene in which the other allele was already inactivated Mutated maternal Normal paternal -/- -/+ Mutated paternal Mutated maternal -/- -/The mutated maternal chromosome was duplicated The presence of one mutated copy increases the chances of a second mutated copy Rb is just one example Inheritance of brca1(lf) mutation results in predisposition for breast cancer Rb = A Tumor Suppressor Gene Retinoblastoma is inherited as a dominant trait, but it is recessive at the cellular level People with familial Retinoblastoma carry one mutated copy in ALL their cells. Cells that would get a second hit will develop Rb or later, other cancers Predisposition is inherited dominantly, but cancer is not inherited The offspring CANNOT inherit two mutated genes How can we clone a tumor-recessive gene? How do we test candidate genes? Oncogenes transform cells into cancerous cells But TSGs are recessive Rb tumors are associated with a deleted region in chromosome 13 Deletion = loss-of-function probably a recessive mutation in the Rb gene Testing a candidate gene Use a fragment of the candidate gene as a probe for Southern Blot analysis Search for absence of the gene in tumors (hoping both mutated copies are deletions) More on this- Angier book, starting p. 334 Rb gene expression is absent or altered in retinoblastoma tumors Rb tumors WT Friend et al. Nature (86) Other tumors Lee et al. Science (87) Northern blots (mRNA expression) We have correlation What about causation? The RB gene is finally cloned Bold Predictions, Further Work Dr. David Abramson, RB expert at New York Hospital (ca. 1986, According to Natalie Angier) “I believe that in fifteen years, at the outside, we’ll be able to stop retinoblastoma before it begins. I’m so sure that I’ve already given the drug a name. I call it retino-revert, or retino-prevent. The drug will be an analogue of the natural protein that is missing in retinoblastoma cells … We’ll be able to diagnose a child prenatally and start giving this retino-revert to the mother to prevent retinoblastomas from growing as the fetus is developing. I know I’m going out on a limb with this one, but … Come back to me in 2001 and tell me if I wasn’t right.” pRb: What does it do? pRb is a nuclear protein that undergoes phosphorylation and dephospharylation in concert with the cell cycle The guardian of the cell at early-mid G1 Hypo-phosphorylated or un-phosphorylated pRb inhibits the cell from entering a new cell cycle Upon further phosphorylation at the R point, hyper-phosphorylated pRb becomes inert and the cell cycle can proceed Hypo-phosphorylated Rb inhibits activity of the E2F family of transcription factors E2Fs are needed for transcription of genes that are essential for the cell to enter the cell cycle Hyper-phosphorylation of Rb sequesters Rb, and releases E2Fs Hypo-phosphorylated Rb binds to E2Fs and: - Inhibits their transcription activation sites - Recruits proteins that will “close” the chromatin down Releasing Rb from the E2Fs leads to: - Release of their transcription activation sites - Recruitment of proteins that will “open up” the chromatin Rb, the retinoblastoma protein regulates the cell cycle Cell cycle = OFF Rb binds to E2F: no transcription, no entry into S phase Cell cycle = ON Rb does not bind to E2F: transcription and entry into S phase w/o 2 copies of Rb: no cell cycle arrest pRb: What does it do? pRb is a nuclear protein that undergoes phosphorylation and dephospharylation in concert with the cell cycle Rb activity is tightly regulated by the cell cycle clock Hypo-phosphorylation is catalyzed by cycD-CDK4/6 Hyper-phosphorylation is catalyzed by cycE-CDK2 pRb is hyper-phosphorylated and inhibited (and released from its role as a guardian), only upon cycE expression Rb activity is tightly regulated by the cell cycle clock However, E-CDK2 can phosphorylate Rb, only AFTER Rb is phosphorylated by cycD-CDK4/6 Have I grown enough? Only after we have enough mitogen signaling (and, as a result, enough cycD-CDK4/6 activity), cycE can phosphorylate Rb and allow entry to the cell cycle E2Fs have more than 100 target genes, mostly involved in the first steps of DNA replication One of the targets: the cycE gene Transcription of cycE starts a positive feedback loop As E2Fs are necessary for expression of cycE, think how critical negative regulation by Rb is for cell cycle control E2Fs Rb gene alteration is involved many tumors In the majority of tumors you will find mutation involved in the R site Uncontrolled crossing of the R site can be due to loss of Rb function (e.g. mutation), loss of CKIs or oncogenic activity of cyclins E and D What not to focus on Details of the cell cycle (e.g. what happens in prometaphase) Molecular details of ubiqu. pre-replicative complex, etc. What to focus on Her-2 Cell cycle control Regulation of CDKs Mitogens and the cell cycle Rb: genetics The restriction point: cycD, cycE, E2Fs, p16 and Rb (read the textbook – chapter 8)