Glycan and disease
advertisement

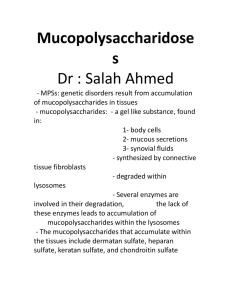
Glycan and disease Classification of human diseases known to be related to glycans • Infectious disease – Bacterial and viral infection – Parasite infection • Genetic disorders – Glycan synthesis/degradation related – Glycosylation related • Acquired diseases • Cancer Polysaccharides on bacterial surface • Polysaccharide capsule – Covers bacterial surface – Targets of immune clearance • Human’s ability to generate antibody responses against bacteria diminished at extremes of age • Certain bacteria avoid antibody defenses through molecular mimicry of common host glycan structure – group A Streptococcus (GAS): capsule of hyaluronan, identical to the nonsulfated glycosaminoglycan – Neisseria meningitidis: homopolymeric sialic acid capsules • Certain pathogens has the great diversity of capsular structures – Can be used to classify different “serotype” strains – Individuals can be repeatedly infected over their lifetime by different serotype strains of the same bacterial pathogen – Genetic exchange of capsule biosynthetic genes among serotype strains of a specific species (e.g., the polysialyltransferase gene in meningococcus) can lead to capsule switching in vivo Glycan adhesins and receptors • Most microorganisms express more than one type of adhesins (mainly lectins) – mediated through terminal sugars or internal motifs. Polymicrobial biofilm • Biofilm formation is a mechanism that promotes bacterial attachment to host surfaces; • Bacteria within biofilms communicate with one another through soluble signaling molecules in a process known as “quorum sensing” to optimize gene expression for survival; • In biofilms, bacteria live under nutrient limitation and in a dormant state in which defense molecules (e.g., antimicrobial peptides) produced by the immune system and pharmacologic antibiotics are less effective; • The excellular polysaccahride (EPS) matrix can bind and inactivate the defense molecules, contributing to the persistence of the biofilm and difficulty in medical treatment of biofilm infections. Polymicrobial biofilm (dental plaque) Bacterial toxins binding to glycans and entering the host cell Proposed receptor sequence Microorganism Toxin Target tissue Bacillus thuringiensis crystal toxins (killing plant-pathogenic insects) Galβ1–3/6Galα/β1– 3(±Glcβ1–6)GalNAcβ GlcNAcβ1–3Manβ1– 4GlcβCer intestinal epithelia of insects (only conserved in invertebrates, from nematode to insects) Clostridium botulinum botulinum toxins (A–E) gangliosides GT1b, GQ1b nerve membrane Clostridium difficile toxin A GalNAcβ1–3Galβ1– 4GlcNAcβ1–3Galβ1– 4GlcβCer large intestine Clostridium tetani tetanus toxin ganglioside GT1b nerve membrane Escherichia coli heat-labile toxin GM1 intestine Shigella dysenteriae Shiga toxin Galα1–4GalβCer Galα1– 4Galβ1–4GlcβCer large intestine Vibrio cholerae cholera toxin GM1 small intestine Mechanism of virus entering the cell Viral infection: glycan-GBP interaction Virus Lectin Glycan receptor specificity Site of infection Influenza A and B (human) hemagglutinin Neu5Acα2–6Gal upper respiratory tract mucosa Influenza A and B (avian and porcine) hemagglutinin Neu5Acα2–3Gal intestinal mucosa Influenza C hemagglutinin-esterase 9-O-acetyl-Neu5Acα- Newcastle disease hemagglutinin-neuraminidase Neu5Acα2–3Gal- Sendai hemagglutinin-neuraminidase Neu5Acα2–8Neu5Ac- Polyoma ? Neu5Acα2–3Gal-, etc Herpes simplex glycoproteins gB, gC, gD 3-O-sulfated heparan sulfate Foot-and-mouth disease caspid proteins heparan sulfate HIV gp120 V3 loop heparan sulfate CD4 lymphocytes Dengue envelope protein heparan sulfate macrophages? mucosal surfaces of mouth, eyes, genital and respiratory tracts Glycan-GBP binding in parasite infection Parasite Stage Protein Specificity Plasmodium falciparum merozoite EBA-175 Neu5Acα2-3Gal/glycophorin A (causing malaria) merozoite EBA-140 sialic acid/glyco phorin B? merozoite EBA-180 sialic acid (erythrocytes) sporozoite circumsporozoite protein heparan sulfate (hepatocytes) trypomastigote trans-sialidase Neu5Acα2-3Gal trypomastigote penetrin heparan sulfate Entamoeba histolytica trophozoite Gal/GalNAc lectin Gal/GalNAc Entamoeba invadens (a reptilian pathogen) cyst cyst wall protein (Jacob lectin) chitin Giardia lamblia trophozoite taglin (α-1 giardin) Man-6-phosphate heparan sulfate Cryptosporidium parvum sporozoite Gal/GalNAc lectin Gal/GalNAc sporozoite Cpa135 protein ? Acanthamoeba keratitis trophozoite 136-kD mannose-binding protein mannose Toxocara canis larval TES-32 ? Haemonchus contortus gut-localized galectin β-galactosides Trypanosoma cruzi Defects in glycoprotein degradation (in lysosomes) Disorder Defect Glycoprotein Glycolipid Clinical symptoms α-Mannosidosis (types I and II) α-mannosidase major none type I: infantile onset, progressive mental retardation, hepatomegaly type II: juvenile/adult onset, milder, slowly progressive β-Mannosidosis β-mannosidase major none severe quadriplegia; mild cases have mental retardation, angiokeratoma, facial dysmorphism Aspartylglucosaminuria aspartylglucosaminidase major none progressive, coarse facies, mental retardation Sialidosis (mucolipidosis I) sialidase major minor progressive, severe mucopolysaccharidosis-like features, mental retardation Schindler (types I and II) α-N-acetylgalactosaminida se yes ? type I: infantile onset, neuroaxonal dystrophy, etc type II: mild intellectual impairment, angiokeratoma, etc Galactosialidosis protective protein major minor coarse facies, skeletal dysplasia, early death Fucosidosis α-fucosidase major minor spectrum of severities includes psychomotor retardation, coarse facies GM1 gangliosidosis β-galactosidase minor major progressive neurological disease and skeletal dysplasia in severe infantile form GM2 gangliosidosis βhexosaminidase minor major slower onset of symptoms and variable symptoms, all relating to various parts of the central nervous system Defects in glycosaminoglycan degradation—the mucopolysaccharidoses Number Common name Enzyme deficiency Glycosamino-glycan affected MPS I H Hurler, Hurler/Scheie, Scheie α-L-iduronidase DS, HS MPS II Hunter iduronate-2-sulfatase DS, HS MPS III A Sanfilippo A heparan N-sulfatase HS MPS III B Sanfilippo B α-N-acetylglucos aminidase HS MPS III C Sanfilippo C acetyl CoA: α-glucosaminide acetyltransferase HS MPS III D Sanfilippo D N-acetylglucosamine 6sulfatase HS MPS IV A Morquio A galactose-6-sulfatase KS, CS MPS IV B Morquio B β-galactosidase KS MPS VI Maroteaux-Lamy N-acetylgalactosamine 4sulfatase DS MPS VII Sly β-glucuronidase DS, HS, CS multiple sulfatase deficiency sulfatase modifying factor converts cysteine→formyl glycine all sulfated glycans Defects in glycolipid degradation Disease name Enzyme or protein deficiency Clinical symptoms Tay–Sachs β-hexosaminidase A severe: neurodegeneration, death by 4 years less severe: slower onset of symptoms, variable symptoms all relating to parts of the nervous system Sandhoff β-hexosaminidase A and B same as Tay–Sachs GM1 gangliosidosis β-galactosidase Sialidosis sialidase Fabry α-galactosidase severe pain, angiokeratoma, corneal opacities, death from renal or cerebrovascular disease Gaucher’s β-glucoceramidase severe: childhood or infancy onset, hepatosplenomegaly, neurodegeneration mild: child/adult onset, no neurodegenerative course Krabbe β-galactoceramidase early onset with progression to severe mental and motor deterioration Metachromatic leukodystrophy arylsulfatase A (cerebroside sulfatase) infantile, juvenile, and adult forms can include mental regression, peripheral neuropathy, seizures, dementia Saposin deficiency saposin precursor similar to Tay–Sachs and Sandhoff Therapies of lysosomal enzyme deficiency • Enzyme replacement therapy (ERT) – Injection of defective enzymes • Enzyme enhancement therapy (EET) – The inhibitors of the enzymes behave as molecular chaperones to stabilize the mutated enzymes in the endoplasmic reticulum to prevent their misfolding and proteasomal degradation – Even though SRT seems promising, the expected benefits to date have been minimal. • Substrate reduction therapy (SRT) – Reducing a glycan’s synthetic rate by can offset the effects of low glycosidase activity Genetic disorder of glycosylations • Inherited disorders occurred in all major glycan synthesis – N-glycan assembly: congenital disorders of glycosylation (CDG) – Galactose metabolism: Galactosemia – Synthesis of the core region of xylose-based GAG chains: Ehlers–Danlos Syndrome – Defects in the formation of heparan sulfate (HS): hereditary multiple exostosis Altered glycosylation in cancer • Increased β1-6GlcNAc branching of N-glycans; • Changes in the amount, linkage, and acetylation of sialic acids; • Truncation of O-glycans, leading to expression of Tn and sialyl Tn antigens; • Expression of the nonhuman sialic acid N-glycolylneuraminic acid, likely incorporated from dietary sources; • Expression of sialylated Lewis structures and selectin ligands; • Altered expression and enhanced shedding of glycosphingolipids; • Increased expression of galectins and poly-N-acetyllactosamines; • Altered expression of ABH(O) blood-group-related structures; • Alterations in sulfation of glycosaminoglycans; • Increased expression of hyaluronan • Loss of expression of GPI lipid anchors.