Blood Clotting
advertisement

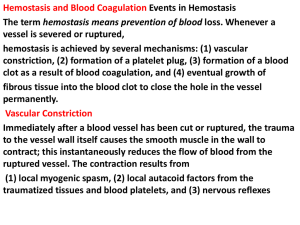
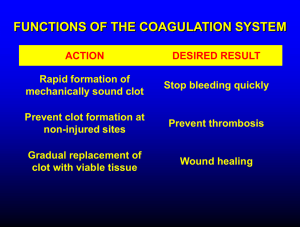
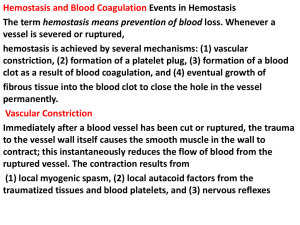
Clotting of Blood Lecture in Basic Tissues 2004 Baynes & Dominiczak, Chapter 6 Gene C. Lavers, Ph.D. gcl1@nyu.edu ©Copyright 1999-2004 by Gene C. Lavers No part of this presentation may be reproduced by any mechanical, photographic, or electronic process, or in the form of a phonographic recording, nor may it be stored in a retrieval system, transmitted, or otherwise copied for public or private use, without written permission from the publisher. Hemostasis (arrest of bleeding) Balancing Events Blood Clotting Introduction Normal hemostasis is a balance between coagulation and fibrinolytic systems. VASOCONSTRICTION PLATELET PLUG FORMATION BLOOD COAGULATION FIBRIN CLOT FORMATION DISSOLUTION OF CLOT PROTECTING THE CLOT PREVENTING CLOT FORMATION © Copyright 1999-2004 by Gene C. Lavers 2 Vasoconstriction Blood Clotting Platelets & Thromboxane Overview VASOCONSTRICTION – occurs immediately after vessel wall injury Diminished blood flow into and out of the vessel. Initiated by local nerve reflexes Sustained through biochemical mediators, serotonin and histamine released from platelets in the damaged subendothelial. Thromboxane A2 (TXA2) – A C20 polyunsaturated fatty acid released from the activated platelets, causes smooth muscle contraction and prolonged vasoconstriction. The larger the portion of vessel traumatized, the more extensive the degree of spasm. A sharply cut vessel (clean cut with sharp razor) undergoes less spasm, i.e., bleeds longer, than a crushed vessel. © Copyright 1999-2004 by Gene C. Lavers 3 Platelet Plug Formation Collagen & von Willebrand Factor Blood Clotting Overview PLATELET PLUG FORMATION – occurs within seconds after vessel wall injury Primary hemostasis, platelets aggregate to control blood loss from capillaries, arterioles, and venules. If damage is small, platelet plug can arrest bleeding completely by closing minute ruptures in vessels that occur hundreds of times a day. Exposure to collagen and other foreign substances (antigen-antibody complexes, thrombin, proteolytic enzymes, endotoxins, and viruses), platelets undergo dynamic change in appearance and begin the process of adhesion and activation. In adhesion, platelets swell, become sticky and adhere to collagen fibril on the basement membrane - requires von Willebrand factor (vWF) adhere to collagen fibrils on the basement membrane (vWF deficiency is associated bleeding disorder). Activated platelets release granules containing vWF, factor V, PDGF*, heparin-neutralizing protein, and ADP (attracts other platelets) * PDGF Platelet-derived growth factor © Copyright 1999-2004 by Gene C. Lavers 4 Clotting Process Blood Clotting Zymogen Cascades Introduction CLOTTING – stops bleeding and hemorrhage. 2 zymogen activation cascades – both convert inactive Stuart factor (X) to active Stuart factor Xa. Xa + Va activates prothrombin (II) to thrombin IIa. Thrombin activates soluble fibrinogen (I) to insoluble fibrin (Ia) Fibrin aggregates forming enormous numbers of fibers that entangle one and other into a meshwork that yields a soft clot. Transglutaminase (XIIIa) cross-links fibrin threads to yield a hard clot. Vitamin K and Ca2+ are essential for liver synthesis of effective protein factors (II, IX, X, XI) that can bind efficiently to membrane phospholipids exposed by wound or trauma. © Copyright 1999-2004 by Gene C. Lavers 5 Clot Prevention/Inhibition Antithrombin III Blood Clotting Introduction INHIBITING CLOT FORMATION Prevention unscheduled clots clots beyond local trauma area. Anti thrombin III (AT III) binds irreversibly to thrombin (IIa). Also binds to IXa, Xa, and XIa of the intrinsic pathway. © Copyright 1999-2004 by Gene C. Lavers 6 Clot Prevention/Inhibition Antithrombin III Blood Clotting Introduction DISSOLUTION OF CLOT – removing unscheduled clots and fostering healing. Tissue Plasminogen Activator (TPA) activates plasminogen to plasmin. Plasmin (a protease) hydrolyzes fibrin to peptides…clot dissolves © Copyright 1999-2004 by Gene C. Lavers 7 Topics-subtopics Blood Clotting Introduction PROTECTING THE CLOT – during healing Antiplasmin inhibits fibrin hydrolysis. PREVENTING CLOT FORMATION – fail safe agent Heparin acts as a catalyst for inactivation of thrombin by antithrombin III. Citrate acts to chelate* Ca 2+ O Oxalate also chelates Ca C 2+ R HO COO– COO– Ca2+ COO– Citrate Chelate: claw Ca2+ C g-carboxylate group C COO– Ca2+ COO– © Copyright 1999-2004 by Gene Oxalate C. Lavers O– O– O 8 Four Principle Components Blood Clotting Introduction Intact endothelial cells – inhibit blood clotting by displaying proteins that inhibit the clotting system, and they form prostacyclin (PGI2) which they release (t/2 ~ 3 min) to prevent platelet aggregation, adhesion, and thrombus formation. Subendothelial tissues – contain membrane proteins normally not in contact with the blood. When exposed after injury, platelets and clotting factors adhere to components of the subendothelial tissue, where they become activated. Platelets – bind to components of the subendothelial tissue. Subsequently, clotting factors bind and are activated on their surface. Clotting factors – are soluble plasma proteins, most are serine proteases, that circulate as inactive zymogens awaiting proteolytic cascade activations that trigger insoluble fibrin formation from soluble fibrinogen. © Copyright 1999-2004 by Gene C. Lavers 9 Blood Clotting Coordinated Four Functions Thrombosis vs. fibrinolysis 4 stages Clotting 3 2 4 1 1. Arteriolar vasoconstriction can transiently reduce blood flow, and can give some platelet aggregation. 2. Activation of blood platelets for adhesion to traumatized vessel wall. 3. Activation of coagulation cascade systems for activation of thrombin. 4. Activation of fibrin polymerization and crosslinking of fibrin soft clot to stable hard clot after vasoconstriction lessens or traumatized tissue is disturbed; clot is anchored. Dissolution peptides 1. 2. TPA activates plasminogen. Plasmin proteolytically digests clot peptides amino acids. © Copyright 1999-2004 by Gene 10 C. Lavers Fig. 6.1 Overview of hemostatic mechanisms Bleeding Disorders Congenital and acquired Blood Clotting Hemophilia Clotting is slow, slower, absent Hemophilia A deficient VIII Hemophilia B deficient IX (Christmas) Fig. 6.2 Congenital and acquired causes of excessive bleeding. © Copyright 1999-2004 by Gene C. Lavers 11 Blood Clotting Classic Hemophilia Factor VIII deficiency Fig. 6.7 Severe bruising from minor fall in a 3-yr old child with classic hemophilia. © Copyright 1999-2004 by Gene C. Lavers 12 Genetic Disorders Blood Clotting Clinical Hemophilia A (recessive, X-linked) - Females carry the defective gene, males express the phenotype of the disorder. 85% of cases, Factor VIII (intrinsic pathway) is missing. Because antihemophilic factor is missing, clotting is blocked. Interaction with Ca-dependent IXa that yields Xa does not occur. Thus, inactve IX fails to act on prothrombin (II) to yield thrombin (IIa). Occurs in about 1 in 10,000 males. Treat with factor VIII or IX concentrates. Hemophilia B - Factor IX (Christmas) is missing, thereby limiting normal blood clotting. © Copyright 1999-2004 by Gene C. Lavers 13 Blood Clotting Vessel Wall Four layered structure Functional Roles Thrombo-resistant endothelial cell lining: produces two vasodilators and inhibitors of platelet function: prostacyclin (prostaglandin I2, PGI2, nitric oxide (NO, also called Endothelium-Derived Relaxing Factor, EDRF) produced from arginine by eNOS (synthase) controlled by intracellular [Ca2+]. Vasoconstriction after vascular injury: mediated in part by serotonin and thromboxane A2 (TXA2) released from activated platelets. Disruption of the endothelial layer exposes subendothelial Tissue factor – activates the extrinsic pathway of blood coagulation. Fig. 6.3 Structure of blood vessel wall. Collagen – activates the intrinsic pathway of blood coagulation. TXA2 is a potent mediator of platelet activation and vasoconstriction Stimulates platelet activation Stimulates platelet binding to exposed collagen via von Willebrand factor (vWF) released from endothelial cells also bound to platelet GPIb-IX receptor. © Copyright 1999-2004 by Gene C. Lavers 14 Blood Clotting Platelet Activation Antiplatelet drugs drugs Eicosanoids Platelets (150-400 x 109/L) Activated by ADP from endothelial, RBC, and Exposed collagen drugs Platelet TXA2 platelets Epinephrine Collagen Thrombin Platelet Activating Factor, PAF Immune complexes (infection) Shear stress from blood flow Platelet shape: disc to sphere Release: TXA2 (t/2, 30sec) and vWF Aggregation via vWF and Exposed collagen fibrinogen binding to GPIbIX and GPIIa-IIIP receptors Adhesion to vessel wall linked by Endothelial lining vWF O COOH CH3 OH © Copyright 1999-2004 by Gene TXA thromboxane 15 Fig. 6.4 Platelet activation 2 Fig 36.16 pathways & antiplatelet drugs C. Lavers Blood Clotting Coagulation Factors zymogens Plasma soluble proteins prothrombin = II IIa = thrombin fibrin I Ia = fibrinogen Intrinsic pathway (•): activated by Clinical tests APTT (30-50 sec) Activated Partial plasma components Thromboplastin Time, also known as the KCCT (kaolin-cephalin clotting time), intrinsic pathway. PT (10-15 sec) Prothrombin time, extrinsic pathway Thrombin time Extrinsic pathway (o): activated by nonendothelial ‘surface’ tissue factor Vitamin K deficiency reduces hepatic synthesis of II, VII, IX, and X. Treat with vit K injections Oral anticoagulents – warfarin reduces hepatic factor synthesis. Excessive bleeding with warfarin treated by stopping the drug, giving vit K or replacing II, VII, IX, and X with fresh frozen plasma or concentrates. Fig. 6.5 Coagulation factors and some of their properties Liver Disease – Factor syn reduced; acetaminophen overdose hepatic failure longer prothrombin time © Copyright 1999-2004 by Gene C. Lavers 16 Topics Blood Clotting Introduction CLOT FORMATION INHIBITING CLOT FORMATION DISSOLUTION OF CLOT PROTECTING THE CLOT PREVENTING CLOT FORMATION © Copyright 1999-2004 by Gene C. Lavers 17 Activation Cascades Intrinsic, Extrinsic, Final pathways Blood Clotting Die XII CO2 Intrinsic Extrinsic Final Prekalikrein HMWK XI XIa Tissue Factor(III) VII + Ca2+ IXa IX Xa X VIII VIIIa XII Prothrombin V thrombin Va XIIa Fig. 6.6 Blood Coagulation fibrinogen © Copyright 1999-2004 by Gene Stable fibrin fibrin C. Lavers 18 Intrinsic and Extrinsic Converge at Xa Blood Clotting Regulation Cascade* Damaged Surface Trauma Damage Intrinsic Pathway Prekallikrein Kininogen Kallikrein Hageman XII Extrinsic Pathway 1 XIIa XI 2 Plasma thromboplastin IX Convertin XIa 3 Stuart VII VIIa Christmas IXa Antihemophilic VIIIa X Preconvertin 4 II Tissue factor Trauma Damage Fibrin X - + - X Xaa Ia IIa Prothrombin Thrombin * Other Cascades? Glycogenolysis cAMP - - I - + - + - + - + - + - © Copyright 1999-2004 by Gene Fibrinogen C. Lavers 19 Activation of Thrombin I IIa NH2 NH2 10 10 Blood Clotting Molecules 274 Arg 275Thr C 274 Arg 275 Thr A NH2 323 Arg 324 Ile S S NH2 A SS C323 Arg B II C 582 324Ile IIa © Copyright 1999-2004 by Gene C. Lavers B C 582 20 Fibrinogen, Factor I –4 –8 Blood Clotting Molecules –4 Properties Synthesized in the liver, t/2 = 4 days 0.3 mg% in human plasma Dimeric heterotrimer: 2 [3 different polypeptides] = [ g]2 joined by S-S bonds, the middle disulfide knot. Each half contains an g subunit mostly in a rod-like triplehelix where the halves join, but with random coils at the outside ends (globular). Fibrinogen is about 9 nm wide and 46 nm long with two halves of 23 nm (230Å). Fibrinogen is trinodular (three-globule dumbbell-shape), central knob has three clusters of charges: -4 at each end and -8 at the central knob: -4 -8 -4 Charge repulsion prevents fibrinogen from self-associating, thus it remains a soluble monomer the circulating blood. © Copyright 1999-2004 by Gene C. Lavers 21 Fibrinogen (I) Fibrin (Ia) Via thrombin (IIa) Blood Clotting Molecules -4 Hydrolysis by Thrombin (IIa) at Arg-gly site yields Fibrin (Ia) monomer + fibrinopeptides A and B Fibrin is insoluble because its - 4 +5 - 4 charges allow it to aggregate to form fibers. The aggregated intertwined fibers trap cells yielding a soft clot. The soft clot is not stable. It is held together by electrostatic charges. Soft clot is cross-linked by factor XIIIa with Ca2+ to yield hard clot fibrin fiber. © Copyright 1999-2004 by Gene C. Lavers -4 +5 22 Hard Clot Formation Transglutaminase (XIIIa) Blood Clotting Cross-linking Gamma chains cross-linked The soft clot continues enlarging as platelets, other blood cells, and debris from the wound, become enmeshed. Factor XIIIa (transglutaminase) cross-links the fibrin monomers comprising the thread-like fibers by forming sets of peptide (amide) bonds between adjoining C-terminal peptides of the g-chains thereby stabilizing and strengthening each fibrous thread along its length. – COOH region: g etc ------------------------Gly Gln Gln His His Leu Gly Gly Ala Lys Gln Ala Gly Asp Val COOH | | COOH Val Asp Gly Ala Gln Lys Ala Gly Gly Leu His His Gln Gln Gly ------------------------------- etc of As cross-linking proceeds, the soft clot becomes more stable and a hard clot develops: clot contracts, water is squeezed out. © Copyright 1999-2004 by Gene C. Lavers 23 g Topics Blood Clotting Outline CLOT FORMATION INHIBITING CLOT FORMATION DISSOLUTION OF CLOT PROTECTING THE CLOT PREVENTING CLOT FORMATION © Copyright 1999-2004 by Gene C. Lavers 24 Antithrombin III Blood Clotting Antithrombin III – always present in the serum. Binding between Antithrombin III and Thrombin (IIa) prevents thrombin from proteolytically activating fibrinogen (I) to fibrin (Ia). Antithrombin III counteracts any unscheduled clot formation. Impact injury triggers clotting at the injury-site by activating thrombin. Any thrombin that might escape the local clot is inactivated, thus, clotting does not spread throughout the body. © Copyright 1999-2004 by Gene C. Lavers 25 Inhibiting Intrinsic Clot Cascade Blood Clotting Process Anti thrombin III binds irreversibly to – IIa (thrombin) active site. – also binds to IXa (Christmas) – also binds to Xa (Stuart) – also binds to XIa (Plasma Thromboplastin) Thus, the active factors exist briefly! * All four have proteins contain N-terminal g-carboxyglutamate clusters with Ca2+ © Copyright 1999-2004 by Gene C. Lavers 26 Inhibitors of Coagulation Blood Clotting Preventing harmful thrombi that cause strokes & infarcts Die CO2 . Fig. 6.8 Sites of inhibitors © Copyright 1999-2004 by Gene C. Lavers 27 Topics Blood Clotting Outline CLOT FORMATION INHIBITING CLOT FORMATION DISSOLUTION OF CLOT PROTECTING THE CLOT PREVENTING CLOT FORMATION © Copyright 1999-2004 by Gene C. Lavers 28 Blood Clotting Coagulation Inhibitors Fibrinolytic System Components Fig. 6.9 Hemostasis Fig. 6.10 © Copyright 1999-2004 by Gene C. Lavers 29 Blood Clotting Fibrinolytic System Plasminogen plasmin Clot Dissolution Fibrin Fibrinogen Degradation Products (FDP peptides) Dissolving clots – Unscheduled Use tPA and streptokinase – Wound healing Plasminogen plasmin(active) Fibrin proteolyized Fibrinogen degradation peptides Saving clots: 2 antiplasmin inhibits plasmin, thus the hydrolysis of fibrin clots is prevented. Fig. 6.11 Fibrinolytic system. Plasminogen to plasmin by uPA, tPA or streptokinase © Copyright 1999-2004 by Gene C. Lavers 30 Topics Blood Clotting Outline CLOT FORMATION INHIBITING CLOT FORMATION DISSOLUTION OF CLOT PROTECTING THE CLOT PREVENTING CLOT FORMATION © Copyright 1999-2004 by Gene C. Lavers 31 Anticoagulants Blood Clotting Molecules Vitamin K Antagonists - Inhibit g-carboxylase thereby lessening g-carboxyglutamate formation. – Dicumarol - present in hay, mimics Vitamin K. – Warfarin (Coumadin)- a clinical anticoagulant (strokes/infarcts). – Chelated Ca2+ normally promotes binding of factors IIa, VIIa, IXa, and Xa to trauma-exposed membrane phospholipids. – The fewer or absence of the g-carboxyglutamate cluster at the Ntermini of these clotting factors slows or prevents these events and interferes with effective clotting. Heparin - A carbohydrate polymer that promotes thrombin inactivation by antithrombin IIIa. Citrate or oxalate - Chelates Ca2+ thereby inhibiting clotting. © Copyright 1999-2004 by Gene C. Lavers 32 END © Copyright 1999-2004 by Gene C. Lavers 33