Chapter 11 - Madeira City Schools
advertisement

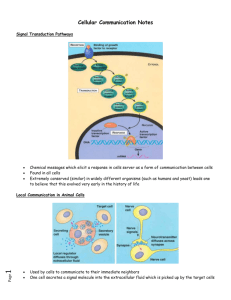
Chapter 11: Cell Communication I. Why do cells communicate? A. Regulation – cells need to control cellular processes Ex: B. Environmental Stimuli – cells need to be able to respond to signals from their environment Ex: II. Stages of cell signaling: A. Reception – signaling molecule binds to a receptor protein in the membrane B. Transduction – passing on the signal (can occur in one step, but is usually a sequence of changes in a series of “relay molecules”) “transduction pathway” C. Response cellular changes because of the signal (catalysis by an enzyme, rearrangement of the cytoskeleton, activation of a specific gene.) III. More Detail A. Reception 1. Signal will only be “heard” by a specific cell. 2. Types of signaling: a. Direct Contact cell junctions; signaling substances dissolved in cytosol are shared (ex: plasmodesmata, gap junctions) membrane bound cell-surface molecules that touch another cell (cell recognition, embryonic development, immuneresponse) b. Local signaling – influence cells in the vicinity Paracrine Signaling Synaptic Signaling c. Long-distance Signaling -- Use Hormones(Endrocrine signaling) Local and Long Distance Signaling Examples Growth Factor Cell secretes molecule (“local regulator”) into extracellular fluid to influence neighboring cells Nervous System More specialized than paracrine. Nerve cell releases a neurotransmitter that stimulates the target cell. Hormones Specialized cells release hormones into circulatory system that carries them to target cells in other parts of the body. AKA – “endocrine signaling” 3. Signal molecules a. often water soluble b. usually too large to travel through membranes c. behave as “ligands” – smaller molecule binding to a larger one. 4. Receptor molecules a. Usually a protein…most are located within the cell membrane (G-protein coupled, tyrosine-kinase, ion channels) b. some are located inside the cell…in the cytoplasm or nucleus of target cell (usually acts as a transcription factor to turn on specific genes). (see slide 15 for more detail) c. Changes shape when bound to signal molecule d. Transmits information from the exterior to the interior of the cell. e. Types of receptor molecules: page 211, 212, 213 G-protein coupled receptor Very widespread and diverse in functions. Ex - vision, smell, blood vessel development. Many diseases work by affecting g-protein linked receptors. Ex - whooping cough, botulism, cholera, some cancers (toxins interfere with G-protein function) Up to 60% of all medicines exert their effects through G-protein linked receptors. Tyrosine-kinase Ion channels Intracellular Receptors G-Protein Coupled Receptor (page 211) Works with the help of a G-protein (protein that binds to GTP) The receptor has a variety of binding sites for different signal molecules and for different G-proteins. There are different “Gproteins” out there. All G-protein coupled receptors have a similar structure. (7 alpha helices spanning the membrane) The “loops” are binding sites for signals and G-proteins Examples: yeast mating factors epinephrine neurotransmitters How the G-Protein Coupled Receptor works (page 211) Works with “G-protein”, an intracellular protein that binds with GDP (G-protein is inactive) or GTP (G-protein is active). When signal binds to receptor, the receptor changes shape…this allows it to bind to an inactive G-protein that is on the cytoplasmic side of the membrane. GTP displaces GDP to activate the G-protein. Now active (GTP is bound to it), the Gprotein binds to an enzyme and alters the enzymes shape and activity. It triggers the next step in a pathway leading to a cellular response. At this point, the G-protein acts as a GTPase enzyme to hydrolyze GTP to make GDP making itself inactive. It is now available for reuse. Tyrosine-Kinase Receptors (page 212) What is Tyrosine? ______________ What is a Kinase? ___________________ Receptor extends through the cell membrane. Intracellular part functions as a “kinase”, which transfers P from ATP to amino acid tyrosine on a substrate protein Often activate several different pathways at once, usually 10 or more (a single binding of signal triggers multiple pathways to occur, helping regulate complicated functions such as cell division and cell growth). Abnormal receptors (meaning they function in the absence of a signal molecule) contribute to some kinds of cancer. How the Tyrosine-kinase receptor works: (page 212) 1. Ligand binding causes two receptor polypeptides to aggregate forming a “dimer” which activates the tyrosine kinase region (inside cell). 2. Each tyrosine kinase adds a P (from ATP) to a tyrosine on the other polypeptide. 3. Now activated, the receptor is recognized by specific relay proteins inside the cell. The relay proteins bind to a specific phosphorylated tryosine causing it to change shape…this triggers a transduction pathway leading to a cellular response. Before signal binds, receptors exist as individual polypeptides 6 6 Ion-Channel Receptors (page 213) Receptors that act as “gates” (open or close in response to chemical signal) that either allows or blocks the flow of ions such as Na+ or Ca2+ through a channel in the receptor. Ex - nervous system signals. neurotransmitter released by one nerve cell to send signal to a neighboring one. It binds to ion channel receptor causing gate to open…letting ions into cell. This triggers an electrical signal (ions are charged atoms) down the receiving cell. There are some gated ion channels that are controlled by electrical signals instead of ligands. These are “voltage-gated ion channels.” Chapter 48 Intracellular Receptors (page 210, then continues on 213) Proteins located in the cytoplasm or nucleus that receive a signal that CAN pass through the cell membrane. Activated protein turns on genes in nucleus. Ex - steroids (hormones) they are lipids, so they can diffuse through if not too big. NO - nitric oxide Enters nucleus with hormone attached Chapter 45 When in the nucleus, the activated receptor protein acts as a transcription factor to turn on a specific gene B. Signal Transduction 1. The further amplification and movement of a signal in the cytoplasm to target molecules. 2. Often has multiple steps using “relay proteins” such as Protein Kinases. a. Remember, a protein kinase is any enzyme that transfers P from ATP to a protein. b. About 2 % of our genes are for Protein Kinases. c. When a protein is phosphorylated, it changes shape and “activates” it. d. Usually adds P to amino acids Serine or Threonine. 3. Protein phosphatases are enzymes that remove phophate groups from proteins. a. this is called “dephosphylation” b. this “inactivates” the protein kinases. c. this provides a way to turn off the signal transduction pathway when the initial signal is no longer present. d. this also makes the protein kinases available for reuse. 4. Secondary Messengers a. Small water soluble non-protein molecules or ions that pass on a signal. b. Spread throughout cell by diffusion. c. Activates relay proteins. d. cyclic AMP (cAMP) and Ca2+ ions are widely used A form of AMP made directly from ATP by Adenylyl cyclase. Enzyme in Short lived -found converted back to AMP. the cell Activates a membrane number of Protein Kinases. Works in response to signal Doesn’t stay in cytoplasm long Figure 11.11 in your book. Page 216 Epinephrine Adenylyl cyclase G Proteincoupled receptor ATP cAMP Protein kinase A Hydrolysis of glycogen e. Calcium Ions More widely used than cAMP. Used as a secondary messenger in both G-protein pathways and tyrosine-kinase receptor pathways. Pathway causes an increase in cytosolic concentration Normally Ca2+ are actively transported out of the cell and imported from the cytosol into the ER, mitochondria, chloroplasts Ca2+ release involves IP3 (inositol triphophate) or DAG (diacylglycerol) Used in plants, animal muscle contraction, secretion of certain substances, and cell division f. Inositol Triphosphate (IP3) and diacylglycerol (DAG) These messengers are made by cleavage of a certain kind of phospholipid in the plasma membrane. Sent to Ca2+ channel on the ER. Allows flood of Ca2+ into the cytoplasm from the ER. C. Cellular Responses 1. Cytoplasmic Regulation a. Rearrangement of the cytoskeleton. b. Opening or closing of an ion channel. c. Alteration of cell metabolism. 2. Transcription Regulation in the nucleus (DNA --> RNA). a. Activating protein synthesis for new enzymes. b. Transcription control factors are often activated by a Protein Kinase. c. can turn a gene on or off. 3. Amplification a. Protein Kinases often work in a cascade with each being able to activate several molecules. b. Result - from one signal, many molecules can be activated because each protein stays in the active form long enough to process many molecules before they become inactive again. 4. Question a. If liver and heart cells both are exposed to ligands, why does one respond and the other not? b. Different cells have different collections of receptors, relay proteins, and proteins needed to carry out the response. 5. Alternate Explanation