Translational Initiation in Eukaryotes
advertisement

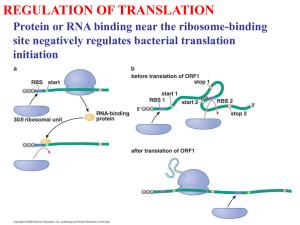
(Lost in) Translation 1. Briefly review prokaryotic machinery 2. Initiation in Eukaryotes 3. Where in the world is Peptidyl Transferase? 4. tRNA charging: The second code 1968 Nobel Prize in Physiology & Medicine (for deciphering the genetic code) “Triumph of the Chemists” H.G. Khorana R. Holley M. Nirenberg Used a cell-free protein synthesis system from E. coli, programmed it with natural and synthetic RNAs of defined sequence, and determined the sequence of the peptides produced. The cell-free system 1. S-30 fraction – ribosomes, tRNAs, tRNA synthetases, other soluble protein factors 2. 20 amino acids 3. GTP & ATP 4. Energy generating system – to keep producing ATP and limit [ADP] – PEP + pyruvate kinase PEP + ADP + Pi pyruvate + ATP 5. Mg2+ and K+ (NH4+) Translation Machinery in Prokaryotes (for comparing with Eukaryotes) • Ribosomes: -70S (composed of L (50S) and S (30S) subunits) -contain 23S (L), 16S (S), and 5S (L) rRNAs -each subunit (L and S) contains ~30 proteins • Initiation factors: if1, if2, if3 • Elongation factors: ef-Tu, ef-Ts, and G • Termination (release) factor(s): Rf1 and Rf2 • Translation is initiated with fmet (N-formylated methionine). How is right AUG selected for translation in Prokaryotes? 1. Many mRNAs contain a sequence preceding the start codon that base-pairs with the 3'-end of 16S rRNA (Shine-Dalgarno sequence) S-D start 5'----GGAGG-------AUG-----3’ mRNA 3'----CCUCC--------5' 16S rRNA 2. 3. 4. Function: helps position mRNA in ribosome. The AUG itself is also very important There is a S-D independent mode of translation initiation in E. coli Translate internal ORFs of polycistronic mRNAs Translational Initiation in Eukaryotes • Begins with methionine that is not formylated • tRNA (tRNAiMet) different from the one that is used for internal methionine codons • Translation start determined by the AUG and surrounding sequence • Translation start site also affected by RNA structure at the 5’ end of the mRNA Scanning Model of Initiation • Proposed by M. Kozak • Small subunit of ribosome (+ initiation factors, GTP and tRNAiMet) binds to the 5’ Cap, and scans along the mRNA until the first AUG • Translation starts at the first AUG • Model seems to work for most mRNAs Scanning (or Kozak) Model for Translation Initiation in Eukaryotes ATP Fig. 17.16 Apparent Exceptions to the Scanning Model? • Translation of some mRNAs (5-10%) doesn’t start at first AUG (ribosome skips one or more AUGs) • Comparative sequence analysis of these mRNAs revealed the following consensus sequence at the AUG that is used: -5 -4 -3 -2 -1 +1 +2 +3 +4 CCRCCAUGG R=purine • Positions -3 and +4 are particularly important, based on mutagenesis studies Effect of the context of an upstream “barrier” ATG on initiation of preproinsulin mRNA. Fig. 17.18 proinsulin Conclusion: When the upstream AUG was in a weak context (like F9), then the downstream one is used. Or, put another way, the first AUG in the right context is used. Upstream ATG is an ineffective barrier if followed by a Stop codon. Stop codon In some mRNAs, the first ATG is in a favorable context, but is still not used. Kozak noted that there was usually a Stop codon in between the start codons in these mRNAs. So she engineered such a situation in the preproinsulin mRNA and tested its affect on translation. Result: Translation was good at the downstream ATG as long as it was in a good context. Fig. 17.23 2nd ed. Conclusions • An upstream AUG does not interfere if it’s context (-3,+4) is poor, or if it is followed quickly by an in-frame Stop codon. - In the latter case, it may be that the ribosomes don’t fall off the mRNA after translating such a short ORF. - In natural mRNAs, upstream ORFs are very short, unless they have a regulatory role. Is the first “good” AUG really favored? Effect of Repeated Initiation Sequences (replicas) AUG AUG AUG Translation started mainly at the first AUG. Fig. 17.19 Effect of RNA Secondary Structure in the 5’ UTR (Leader) Poorly translated Translated well Not translated Trans. well Adapted from Fig. 17.20 Conclusions • Secondary structure (hairpin) at very 5’ end of RNA can prevent 40S subunit from binding • Scanning ribosomes can melt out some hairpins ( ΔG= -30 kcal/mole), but not highly stable ones ( ΔG= -62 kcal/mole) • Initiator tRNA (tRNAiMet) also important in recognizing AUG – (yeast) Anticodon of tRNAiMet changed to UCC, translation started at first “good” AGG in his4 mRNA (Fig. 17.21). Summary of translation initiation in Eukaryotes. Resists binding to 60S subunit Fig. 17.22 Initiation Factors (except eIF-4) eIF-1(and 1A): promotes scanning *eIF-2: binds tRNAiMet to 40S subunit, requires GTP (which gets hydrolyzed to GDP) eIF-2B: catalyzes exchange of GTP for GDP on eIF-2 *eIF-3: binds to 40S subunit, prevents 60S subunit from binding to it eIF-5: stimulates 60S subunit binding to the 48S pre-initiation complex *eIF-6: binds to 60S subunit, helps prevent 40S subunit from binding to it * prokaryotic counterpart eIF4 (eIF4F) eIF4F Originally isolated based on its ability to bind the Cap-nucleotide 7MeGTP. Composed of 3 subunits, a 24-kDa protein that binds the Cap, and 2 others that stabilize the complex and have other roles: 1. eIF4G - versatile adaptor 2. eIF4A - RNA helicase 3. eIF4E - binds the Cap Fig. 17.25 eIF4A and eIF4B eIF4A • also exists outside of the eIF4F complex • contains a DEAD motif (aspartate-glutamate-alanineaspartate) characteristic of RNA helicases • RNA helicase activity was demonstrated (right panel) and found to require ATP and to be stimulated by another protein, eIF4B eIF4B • binds RNA, stimulates eIF-4A Role in translation: Unwind hairpins in the 5’ UTRs 17.26 eIF4G – helps recruit 40S subunit to mRNA; can interact with eIF4E, eIF4A, eIF3, and poly-A binding protein (Pab1); may be responsible for the synergistic effect of Cap and polyA-tail on translation. Similar to 17.27c Why interact with both Cap and polyA-tail? Observation: Some viral mRNAs (such as Polio virus) are not capped, yet are preferentially translated. Some are also translated via internal ribosome entry sites (IRES) (apparently without scanning to them). Mechanism: Viral protease clips off N-terminus of eIF4G, so it can’t bind eIF4E. eIF4G binds a viral protein (X), that binds to the IRES, promoting translation of the uncapped viral mRNAs. 17.27 eIF1 & eIF1A • Genes essential in yeast • Needed for the 40S subunit-particle to scan more than a few nucleotides from the Cap and form the 48S complex • Also dissociate improperly formed complexes between the 40S subunit and mRNA Toe-printing assay for determining where the leading edge of a ribosome (or ribosomal subunit) is on a mRNA Fig. 17.31 RESULTS: formation of Complex II, which is the toe print of the 40S subunit that has scanned to the AUG, is obtained only when eIF1 + eIF1A, or a fraction containing them (50-70% A.S.), was added. eIF1+eIF1A also convert Complex I into Complex II when added after Complex I has formed (lane 8). Fig. 17.32