EN_2ndW_2013_L
advertisement

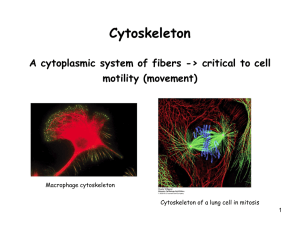
Non-membranous cell organelles. Cytoskeleton – structure, function and tissue specificity. Specialization of the cell surface. Intercellular junctions. Biological motors – molecular principles Institute of Histology and Embryology Author: Prof. MUDr. Jindřich Martínek, DrSc. Subject: General Histology and general embryology Code: 82241 th th Date: 2013, October, 10 and 12 NON-MEMBRANOUS CELL ORGANELLS • • • • NUCLEOLUS RIBOSOME CENTRIOLE CYTOSKELETON thin filaments - actin intermediate filament lamins (A, B, C) cytokeratins (1 – 20) desmin, vimentin nestin microtubules Becker: The world of the cell, 1986 NON-MEMBRANOUS CELL ORGANELLES • • • • NUCLEOLUS – localization in the karyoplasm – nucleolar organizing centers (NORs) two components (amorphous and granulous) – site of transcription of r-RNAs (ribosomal) – compact, network and ring-form type CENTRIOLE – specific organization of microtubeles (9 triplets) – MTOC – microtubular organizing center function, simple and multiple centriolar replication. Role in formation of mitotic and meiotic spindle and basal bodies of cilia and flagella CYTOSKELETON – complex of thin (5-7 nm) and intermediate (10-15 nm) filaments and microtubules (24 nm) – actin (thin) filaments with myosin and tubulins (of microtubules) with dynein and kinesin represent molecular motors for intracellular traffic – intermediate filaments are nonpolar – nestin, cytokeratins, vimentin, desmin – tissue specific – cells of epithelial origin (cytokeratin), mesenchymal origin (desmin), muscle tissue (vimentin) RIBOSOMES – (15-30 nm) composed of two subunits (small and large) – play a role in translation – protein synthesis – as free polysomes and membrane bounded (GER) NUCLELUS – non-membranous cell organelle derived from activated parts of some chromosomes – nucleolar organizing regions (NORs) or nucleolar organizing centers (NOCs) Types: compact – initial transcription of rRNA at the single NORs; network – massive transcription in gradually confluent NORs ring-form – decreasing transcription at the single NOR FC = fibrilar center – NOC PF = pars fibrosa – accumulated transcript PG = pars granulosa – ribosomal assembly PNC = perinucleolar chromatin PNC PF PG FC PNC VISUALISATION OF NUCLEOLAR ORGANISERS USING IMMUNOHISTOCHEMICAL PROBES 1 2 3 4 2 3 1 TYPES OF NUCLEOLI: a) compact – currently activated nucleolar organiser b) with nucleolonema – massive formation of ribonucleoproteins around NORs - typical network appearance c) ring form – decreased of RNA transcription in the inactivated nucleolar organiser 1 = perinucleolar chromatin 3 = nucleolonemata 5 = lamelae annulatae 2 = nucleolar organiser 4 = nuclear channel system 6 = lysosomes 5 6 RIBOSOMES (15 – 30 nm) free polysomes GER PROTEIN SYNTHESIS NUCLEUS NUCLEAR PORES GER GER RIBOSOME – TRANSLATION MECHANISM – POLYSOMES Start codon (AUG) Currently translating codon (arbitrary) Scheme of the protein synthesis on free polysomes (Junqueira´s Basic Histology, Mesher, 2010) ribosome GER GER mRNA free protein in the cytoplasm Electron micrograph: Histology, Ross, Pawlina 2010 Arrows: free polysomes Rough (Granular) Endoplasmic Reticulum rER (GER) GER IMMUNOHISTOCHEMICAL DETECTION OF SOME CYTOSKELATAL COMPONENTS Microtubules (red), actin filaments (green)– fluorescence microscope CHARACTERISTICS OF CYTOSKELETAL COMPONENETS Ross, Pawlina: Histology, 2006 Actin filaments Diameter: 6 – 8 nm Composition: Polymer of G-actin Intermediate filaments Structure: Double-stranded F-actin helix Thin flexible filament Readily dissociate and reassamble Enzyme activity: ATP hydrolitic activity ATP-dependent polymeration Location and function in the cell: Terminal web Zonula adherens Core of microvilli Contractile ring in the dividing cell Contractile elements of muscles Microtubules 10 – 12 nm Various proteins Ropelike fiber Strong, stable structure 24 nm Dimers of α- and ß-tubulin Hollow non-branched cylinder Exhibit dynamic instability None Extend across cytoplasm connecting desmosomes and hemidesmosomes Nuclear lamina of nucleus Support of cell processes Provide mechanical strenght and resistence to shearing forces GTP hydrolytic activity GTP-dependent polymeration Core of cilia and flagellum Centriole Mitotic spindle Provide network “railroad tracks“ for movement of organelles within cell Movement cilia and chromosomes (during cell division ACTIN thin filaments (5 – 7 nm) G and F actin – ATP dependent (poly- and depolamerization) MOLECULAR MOTOR COMPONENT together with myosin Smooth muscle cells Hearth muscle cells - cardiomyocytes FUNCTION OF MICROFILAMENTS AND MICROTUBULES IN REGULATIONS AND INTRACELLULAR TRAFIC THE MOLECULAR MOTOR PROTEINS WORKING WITH MICROFILAMENTS (ACTIN) Unipolarly organized myosin (myosin monomers – myosin I) – one way movement of cargo Bipolar organization of myosin (myosin monomers – myosin II) – ontractile activity with opposite direction movement of actin filaments – muscle tissue Unipolarly working myosin (I) – attached to the cytoplasmic membrane – formation of pseudopodia movement of cell THE MOLECULAR MOTOR PROTEINS WORKING WITH TUBULINS OF MICROTUBULES Kinesins move along the MT tothe plus end and can transport cargo (organelles) from the cytocentrum toward the cell periphery Dyneins move along the MT to the minus end, transport cargo (endocytotic vesicles) from the cell Scheme: Histology, Ross, Pawlina, 2010 periphery toward the MTOC. INTERMEDIATE FILAMENTS – non-polar lamins A, B, C – nuclear lamina cytokeratins – cells of germ layer origin desmin – cells of mesenchymal origin vimentin – cells of mesenchymal origin neurofilaments – nerve cells GFAP – glial fibrillary associated protein (acidic) nestin – during cell development CYTOKERATIN 18 – fission products – APOPTOTIC MARKER MICROTUBULES (25 nm) tubulin α, β, γ (GTP – dependent polymerization) MOLECULAR MOTOR COMPONENT – together with dynein (+ -) and kinesin (- +) CENTRIOLAR PAIR - CENTROSOME CILIUM – KINOCILIUM Ross, Pawlina: Histology, 2006 CENTRIOLES AND CENTRIOLAR REPLICATION Centriolar pair = diplosome – centrosome Centriole: Procentriole: = 200 nm = 200 nm L = 400 nm L = 200 – 400 nm Centriolar DNA – MTOC – centriolar precursors SIMPLE REPLICATION – begins from existing centriolar pair MULTIPLE REPLICATION – starts as synthesis of precursor material (tubulins) and continues as induced assembly of nine procentriolar triplets of microtubules – typical for cells with kinociliary apparatus at the apical surface ZONULA OCCLUDENS – tight junctions – missing intercelluar space Mucus layer ZONULA OCCLUDENS – freeze fracturing technique Ridges Grooves Ciliary necklaces Ross, Pawlina: Histology, 2006 Golgi complex Zonula occludens Zonula adherens Desmosome – macula adherens Cytokeratin – intermediate filaments ZONULA ADHERENS – terminal web – transverally arranged actin filaments inserting via α-actinin and vinculin and catenin into E-cadherin moleculs GAP JUNCTIONS – NEXUSSES – communicative cell to cell interconnections connexon – canal formation connexins – membrane proteins MICROVILLI = 0.1 m lenght = 1 – 5 m – core – actin filaments Regularly arranged and numerous microvilli represent specific surface specialization of an absorptive epithelium – brush border – at the apical pole of cells. Stereocilium – long and branched microvillus Ross, Pawlina: Histology, 2006 Microvilli at the cell suface and intercellular interconnection ZO = zonula occludens; ZA = zonula adherens; D = desmosome zo ZA D SOLITARY (single or individual) CILIUM = 0.25 m L = 3 – 5 (7) m (50 for tail of spermatozoon) Consist of basal body (9 triplets – MTOC) and axonemal complex (9 doublets and 1 central pair of microtubules). Basal body develops from one of centrilar pair and therefore it is located often in the CYTOCENTRUM. Pinocytotic vesicle Ciliary sheat Basal body Axonemal complex Ross, Pawlina: Histology, 2006 KINOCILIARY APPARATUS Oviduct – simple columnar ciliated epithelium Cilia and microvilli at the cross section CILIA, MICROVILLI, JUNCTIONAL COMPLEXES AND TERMINAL WEB INTERDIGITATIONS IMMUNOFLUORESCENT DETECTION OF PANCADHERIN IN SEMINIFEROUS TUBULES OF TESTIS (Zonulae adherentes) BETWEEN SERTOLI CELLS PROCESSES BASOLATERAL LABYRINT Typical for absorptive type of epithelial cells as in the proximal tubules (kidney) or in lining of so called striated ducts in some salivary glands. Specific for an intesive Ion transport (energy needed) by e.g. Na+, K+ ATP-ase as an integral protein of the plasma membrane Stevens, Lowe: Histology, 1993 Ross, Pawlina: Histology, 2006 Stevens, Lowe: Histology, 1993