Plasma Lipoproteins
advertisement

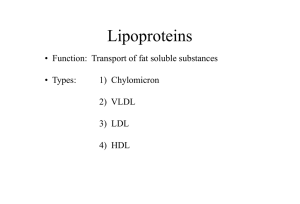
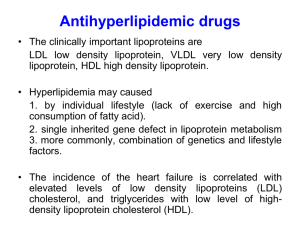
Plasma Lipoproteins 1. 2. 3. 4. What are plasma lipoproteins? Spherical macromolecular complexes of: Lipids + specific proteins (apo-proteins) They includes: Chylomicron Very low density lipoproteins (VLDL) Low density lipoproteins (LDL) High density lipoproteins (HDL) How can lipoproteins Differ? 1. 2. 3. They differ according to: Composition of lipids to proteins Size Density Functions of Lipoproteins Transport lipids in plasma by the protein portion (keep lipids soluble) Transporting their lipid content to & from tissues N.B. In humans, the transport system is less perfect than in other animals cholesterol deposition in tissues atherosclerosis Composition of Plasma Lipoproteins Neutral core (TAG, exogenous or de novo, cholesterol esters) Amphipathic apolipoprotein Phospholipids Cholesterol Size & Density Chylomicrons: largest in size, lowest in density, highest in lipids & lowest in proteins VLDL & LDL: denser, higher ratio of protein to lipid than chylomicrons HDL: densest N.B. lipoprotein particles constantly interchange lipids & proteins with each other making them variable N.B. lipoproteins can be separated by electrophoresis (mobility) or by ultracentrifugation (density) Apolipoproteins The apolipoproteins associated with lipoprotein particles have a number of diverse functions Proteins in nature Function: 1.has recognition site for cell-surface receptors 2.serve as activators or coenzyme for lipoprotein metabolism 3.important for lipoprotein function 4.Some are freely transferred between lipoproteins Classes of apolipoproteins A, B, C, D, E are major classes Subclasses: apo A-1, apo C-II N.B. function of all apolipoproteins are not yet known Metabolism of Chylomicrons CM Site: intestinal mucosal cells Function: carry dietary TAG, chol., fat-soluble vit., chol. esters & lipids made in intestinal cells to peripheral tissues. Synthesis of apolipoproteins : Apo B-48 is unique to CM Synthesized in rough endoplasmic reticulum (RER) Named 48 because 48% of n-terminal protein is coded by apo B gene Composition of CM Apoprotein Apo B-48 Apo C-II from HDL Apo E from HDL Assembly of CM: Requires microsomal TAG transfer protein to load apo B-48 with lipids (TAG, Chol. phospholipids) Synthesis occurs in ER golgi packed in secretory vesicles fused with plasma membrane releasing lipoproteins lymphatic system blood Modification of Nascent CM Intestinal mucosal cells produce nascent CM Called nascent because it is functionally incomplete Once reaches plasma, CM particles receive apo E & apo C-II from HDL Apo C-II is activator of lipoprotein lipase that degrades TAG in CM Formation of CM Remnant As CM circulates & its TAG degrades by lipoprotein lipase, apo C is returned to HDL remnant CM liver where its cell membrane can recognize apo E (receptor) to take up CM remnant by endocytosis degradation releasing AA, cholesterol & FA Lipoprotein lipase Lipoprotein lipase is extra-cellular enzyme of capillary walls of adipose tissue, cardiac & skeletal muscle, not found in liver Activated by apo C-II Hydrolyzes TAG in CM FA + glycerol FA either stored by adipose tissues or generate energy by muscle Glycerol liver for lipid synthesis, glycolysis or gluconeogenesis Type 1 hyperlipoproteinemia or familial lipoprotein Lipase deficiency Patients deficient in lipoprotein lipase or apo C-II Accumulation of CM in plasma N.B. lipoprotein lipase is stimulated by insulin Metabolism of VLDL Site of production: Liver Composition: predominately TAG, apo B-100& obtain apo C-II & apo E from circulating HDL Function: carry TAG from liver to peripheral tissues apo C-II is required for activation of lipoprotein lipase Modification of circulating VLDL TAG is degraded from VLDL by lipoprotein lipase particles decrease in size & gets denser Apo C & E return to HDL TAG goes to HDL & Cholesterol ester (CE) from HDL goes to VLDL (exchange mechanism by CE transfer protein) fig18.18 Production of LDL from VLDL VLDL in plasma LDL + IDL (VLDL remnant) IDL can be taken up by cells via receptor-mediated endocytosis using apo E (apo E isoforms: E2, E3, E4) Apo E2 binds poorly to receptors Metabolism of VLDL In peripheral tissues, VLDL-TAG are digested by LPL, and VLDL is converted to IDL. IDL returns to the liver, is taken up by endocytosis, and is degraded by lysosomal enzymes. IDL can also further degradation, forming LDL. LDL reacts with receptors with receptors on various cells and is digested by lysosomal enzymes. A beta lipoproteinemia Hypolipoproteinemia Defect in TAG transfer protein Inability to load apo B with lipid No chylomicrons or VLDLs TAG accumulates in liver & intestine Familial Type III hyperlipoproteinemia Also called familial dysbetalipoproteinemia Patients are deficient in apo E2 Clearance of CM remnant & IDL Hypercholesterolemia Metabolism of LDL Composition: 50% cholesterol & CE, apo 100 Function: provide cholesterol to peripheral tissues or return it to liver Mechanism of LDL uptake: by cell-surface membrane LDL receptors that recognize apo B-100 (LDL) or apo E (VLDL) These receptor are called apo B-100/apo E receptors The primary function of LDL particles is to provide cholesterol to the peripheral tissues (or return it to the liver). LDL receptors are negatively charged glycoproteins that are clustered in pits on cell membranes. The intracellular side of the pit is coated with the protein clathrin, which stabilizes the shape of the pit After binding, the LDL-receptor complex is internalized by endocytosis The pH of the endosome falls (due to the proton-pumping activity of endosomal ATPase), which allows separation of the LDL from its receptor. The receptors then migrate to one side of the endosome, whereas the LDLs stay free within the lumen of the vesicle. [Note: This structure is called CURL Compartment for Uncoupling for Receptor and Ligand] The receptors can be recycled, whereas the lipoprotein remnants in the vesicle are transferred to lysosomes and degraded by lysosomal (hydrolytic) enzymes, releasing free cholesterol, amino acids, fatty acids, and phospholipids. These compounds can be reutilized by the cell. LDL Receptors -ve charged cell membrane glycoprotein Intracellular side is coated with protein clathrin to stabilize the receptor LDL receptor deficiency plasma LDL & cholesterol type II hyperlipidemia (familial hypercholest.) T3 cause +ve effect on LDL binding to its receptors Hypothyroidism hypercholesterolemia LDL Receptors (continue) Vesicle containing LDL loses its clathrin & fuses with other vesicles large vesicles endosomes pH of endosomes due to proton pump ATPase CURL LDL + free receptor N.B.CURL is Compartment for Uncoupling of Receptor & Ligand Receptor can be recycled (fig 18.20) Effect of endocytosed cholesterol on cellular cholesterol homeostasis The chylomicron remnant-, IDL-, and LDLderived cholesterol affects cellular cholesterol content in several ways. First, HMG CoA reductase is inhibited by high cholesterol, as a result of which, de novo cholesterol synthesis decreases. Second, synthesis of new LDL receptor protein is reduced by decreasing the expression of the LDL receptor gene, thus limiting further entry of LDL cholesterol into cells Effect of endocytosed cholesterol on cellular cholesterol homeostasis Third, if the cholesterol is not required immediately for some structural or synthetic purpose, it is esterified by acyl CoA cholesterol acyltransferase (ACAT) transfers a fatty acid from a fatty acyl CoA derivative to cholesterol, producing a cholesteryl ester that can be stored in the cell. The activity of ACAT is enhanced in the presence of increased intracellular cholesterol. Macrophage Scavenger receptors In addition to the highly specific and regulated receptor-mediated pathway for LDL uptake, macrophages possess high levels of scavenger receptor activity. These receptors, known as scavenger receptor class A (SR-A), can bind a broad range of ligands, and mediate the endocytosis of chemically modified LDL. Macrophage Scavenger receptors Chemical modifications that convert circulating LDL into ligands that can be recognized by SR-A receptors include oxidation of the lipid components and apolipoprotein B. Unlike the LDL receptor, the scavenger receptor is not down-regulated in response to increased intracellular cholesterol. Cholesteryl esters accumulate in macrophages and cause their transformation into "foam" cells, which participate in the formation of atherosclerotic plaque Macrophage Scavenger receptors Receptor: scavenger receptor class A (SR-A) Ligand: oxidized LDL (lipid + apo B) SR-A is not down-regulated to increased intracellular cholesterol cholesterol in macrophages foam cells atherosclerosis plaque (fig 18.22) Lipoprotein (a) in heart disease: Is identical to LDL & linked to apo B-100 It increases the risk for coronary heart disease Metabolism of HDL (good Cholesterol carrier) Heterogeneous, secreted into blood from liver & intestine Structure: nascent HDL contains PL, apo A,C & E fig.18.23 N.B PL solubilize cholesterol Function: 1. reservoir of apolipoproteins apo C-II VLDL & chylomicrons, apo E is required for receptor-mediated endocytosis of IDLs & chylomicron remnants 2. activator of lipoprotein lipase 3. Uptake of cholesterol from other lipoproteins & cell membrane 4.Esterification of Chol. in HDL by plasma phosphatidylcholine : chol.acyltransferase (PCAT) [also known as LCAT L for lecithin] LCAT binds to nascent HDL & is activated by apo A-1 5.Selective transfer of chol. from peripheral cells to HDL & from HDL to liver for bile acid & hormone synthesis mediated by scavenger receptor class B-1 (SR-B1) key for chol. Homeostasis, plasma HDL atherosclerosis Metabolism of HDL (good Cholesterol carrier) HDL is a reservoir of apolipoproteins: HDL particles serve as a circulating reservoir of apo C ll (the apolipoprotein that is transferred to VLDL and chylomicrons, and is an activator of lipoprotein lipase), and apo E (the apolipoprotein required for the receptor-mediated endocytosis of IDLs and chylomicron remnants Apo C ll and Apo E are transferred back to HDL following digestion of TAG of chylomicrons and VLDL. HDL uptake of unesterified cholesterol: Nascent HDL are disk-shaped particles containing primarily phospholipid (largely phosphatidylcholine) and apolipoproteins A, C, and E. They are rapidly converted to spherical particles as they accumulate cholesterol. [Note: HDL particles are excellent acceptors of unesterified cholesterol (both from other lipoproteins particles and from cell membranes) as a result of their high concentration of phospholipids, which are important solubilizers of cholesterol.] Esterification of cholesterol: When cholesterol is taken up by HDL, it is immediately esterified by the plasma enzyme phosphatidylcholine:cholesterol acyltransferase (PCAT, also known as LCAT) This enzyme is synthesized by the liver. PCAT binds to nascent HDLs, and is activated by apo A-1 PCAT transfers the fatty acid from carbon 2 of phosphatidylcholine to cholesterol. This produces a hydrphobic cholesteryl ester. As cholesterol esters accumulate in the core of lipoprotein HDL transfers cholesterol esters to other lipoproteins in exchange for various lipids. Cholesterol ester transfer protein (CETP) mediates this exchange HDL and other lipoproteins carry the cholesterol esters back to the liver. Activity Schematic diagram of lipogenesis and lipolysis Schematic diagram of ketogenesis and ketolysis Schematic diagram cholesterol synthesis Discuss types and functions of plasma lipoproteins.